Otilia Anca Tica1, Ovidiu Tica2,3, Romina Tor1, Adrian Hatos4, Ioana Cote1, Mihnea Nichita Brendea1, Larisa Rosan1, Livia Mihele1, Madalina Moisi1, Mircea-Ioachim Popescu1,3
1 Clinic of Cardiology, Emergency County Clinical Hospital, Oradea, Romania
2 Department of Pathology, Emergency County Clinical Hospital, Oradea, Romania
3 Faculty of Medicine and Pharmacy, Oradea, Romania
4 Faculty of Social-Humanistic Sciences, University of Oradea, Romania
Abstract: Introduction – Heart failure (HF) and atrial fi brillation (AF) coincide in many patients. The bond between these two conditions is sealed by the shared similar risk factors and common pathophysiology. Purpose – The objective of our study is to assess the prevalence, clinical characteristics and to determine in-hospital mortality of non-valvular AF in HF patients. Methods: A total of 434 patients admitted consecutively in our clinic with diagnosis of AF and HF were evaluated during hospitalization. Baseline characteristics and clinical outcomes were extracted. The patients were divided in two gropus: valvular and non-valvular AF. Results – The mean age of our studied group (263 eligible patients with non-valvular AF and HF) was 73.79 years with a SD of 10.487 (p=0.000). Comorbidities found among our patients were: anxiety disorders (37.3%), chronic kidney disease (31.2%), diabetes mellitus (28.1%), arthrosis (28.1%), hepatic disorders (25.5%), obesity (24.0%), malignancy (22.5%), left bundle branch (12.2%), Parkinson disease (9.9%), hemorrhagic events (8.7%), stroke (8.4%), peripheral vascular disease (7.2%), anemia (6.8%), and right bundle branch (5.3%). Conclusion – The presence of non valvular AF in HF patients is associated with a high number of risk factors, comorbidities and high in-hospital mortality. Keywords: atrial fibrillation; heart failure; anticoagulants; comorbidities; mortality
INTRODUCTION
Atrial fibrillation (AF) is the most common arrhyth-mia worldwide. AF is still one of the leading causes of heart failure, stroke, sudden death, and cardiovascular morbidity in the world1, although major progresses were made in the management of patients with this arrhythmia.
It is estimated that its prevalence is 3% in adults aged 20 years or older2,3, with a greater value in el-derly patients4. AF is independently associated with a two-fold increased risk of all-cause mortality in wo-men compared to men5 (a 1.5-fold increase) and has increased morbidity6,7. In developing countries, the age-adjusted incidence and prevalence of AF are lower in women, while the risk of death in women with AF is similar to or higher than that in men8,9.
Heart failure (HF) and AF coincide in many patients. The bond between these two conditions is sealed by the shared similar risk factors and common pathophy-siology. Structural cardiac remodling, activation of neurohormonal mechanisms, and rate-related impair-ment of left ventricular (LV) function are some of the incriminated causes and exacerbation between these two nexus10. Both conditions interact with each other causing increased mortality rates.
AF and HF are cardiovascular disease epidemics that have grown worldwide in the past 2 decades11.
The interaction between HF and AF has been a qu-est for many researchers12-14.
AF has many underlying cardiovascular diseases and can be precipitated by concomitant conditions. In or-der to prevent AF burden, an important keystone, is treating, preventing and certifying15,16 these factors.
It is a challenge to diagnosis AF, especially silent episodes, before it’s redoubtable complications are installed (stroke and decompensated heart failure). The major goals of AF imply rate and rhythm control, stroke prevention therapy, acute management, treat-ment of underlying and concomitant cardiovascular conditions. In the last years a great effort throughout countless research and studies17 were made, but ne-vertheless, there are still gaps to be covered concer-ning treatment options, and AF management.
Due to high prevalence and important impact, HF prevention requires tilted attention towards affected AF patients. Therefore, it is extremely important to identify clinical aspects of AF and HF patients. A com-mon group of patient encountered in daily practice is the one that combines heart failure and atrial fibrillation.
AIM
The objective of our study is to assess the prevalen-ce, clinical characteristics and to determine in-hospital mortality of non-valvular AF in HF patients.
METHOD
Study population
We conducted a retrospective observational study among adult patients, who have the clinical diagnosis of AF and HF. A total of 263 patients admitted conse-cutively in our hospital between January 2016 and De-cember 2016, were evaluated. Baseline characteristics and clinical outcomes were extracted. The main inclusion criteria for study participation was documented AF and HF. Patients admitted in another clinic or de-partment and transferred to ours, were also included. Patients with exclusively short, temporary AF episo-des (e.g. AF following cardiac surgery) were excluded. Patients suffering from an acute disease, other than the cardiac one (e.g. general surgery), or patients that were transferred from our clinic to another were not enrolled.
Assessments
Data were obtained from the patient’s medical charts; the demographic information, as well as clinical assess-ment and comorbidities were noted. Laboratory fin-dings in conjunction with physician notes as well as medication from the patient’s medical charts, were used to determine whether or not they have a specific comorbidity. CHA2DS2-VASC and HAS-BLED scores were assessed for each patient and noted.
Patients were divided in two groups: valvular and non-valvular AF. Valvular AF according to the Euro-pean Society of Cardiology Guidelines defi nition includes patients with moderate to severe mitral stenosis or prosthetic heart valves (and valve repair in North American guidelines), and thus they should be treated with VKA. Valvular heart diseases, such as mild mitral stenosis, mitral regurgitation, aortic stenosis and aor-tic insufficiency, do not alter the low flow in the left atrium, and it seems they do not increase the risk of cloth (induced by AF).
Arterial hypertension was defined on the basis of clinical history or by the use of antihypertensive me-dication at admission. Congestive heart failure and cardiomyopathy were diagnosed according to the European Society of Cardiology (ESC) definition. The diagnosis of ischemic heart disease was made on the patient’s history of significant coronary artery disease revealed by coronary angiography or on the basis of chest pain associated with elevated level for cardiac markers (troponin I or high sensitivity troponin I) / echocardiography changes consistent with the valida-ted ischemia on the electrocardiography, or a positi-ve non-invasive stress test. The diagnosis of valvular heart disease was established by moderate or severe valvular stenosis or regurgitation. Diabetes was ascer-tained by a fasting serum glucose value greater than 126 mg/dl, a HbA1c greater than 6.5% or the use of glucose lowering agents or insulin. The diagnosis of chronic kidney disease was determined by a creatinine clearance calculated by MDRD study equation lower than 60 ml/min/m2. Ischemic or hemorrhagic stroke were certified by a cerebral computer tomography scan (performed during admission or in emergency department) and neurological assessments. Chronic obstructive pulmonary disease was set out by abnor-mal pulmonary function tests or current treatment with an inhaled long acting bronchodilator and/or an inhaled corticosteroid. Endocrine disorders assessed were: pituitary, thyroid disorders (estimated throu-ghout TSH level, free T4 and/or T3 value); adrenal disorder (searched in patients that hade an intake of ≥7.5 mg prednisone equivalent); pheocromocitoma (take into consideration in patients with high levels of catecholamines); primary aldosteronism (considered in patients with high aldosterone levels). Anemia was considered as a reducing amount of red blood cells (RBCs) per mm3 of blood, or a decrease in hemoglo-bin value (below 13 g/dL in men and under 12 g/dL in women). Patients who met the inclusion criteria but died during the specified observation range were also included in the study.
Statistical analysis
All statistical analyses were conducted using SPSS 21. Results are presented as mean ± standard deviation SD (for numerical variables) or percentages. Conti-nuous variables were reported as the mean±SD or as the median and interquartile range (IQR). Categorical variables were reported as percentages. Continuous variables were analyzed for normalization and compa-red using the t Student test; they were expressed by mean value ± standard and/or median deviation. For comparison of parameter averages the Mann-Whitney U method and the Wilcoxon method W are used. The degree of correlation (r) between the studied para-meters was evaluated by calculating the correlation coefficient Pearson. On multivariate analysis, logistic regression model was used. A cut-off value of p <0.05 was considered statistical significant. Intergroup com-parisons were made using a Chi-square test.
RESULTS
From a total of 434 patients admitted within one year into our hospital, a group of 92 patients were excluded from the study due to missing data or they were lost-to follow-up. Patients were divided in two groups: valvular (79 patients with a SD of 0.294) and non-valvular (263 patients with a SD of 0.299) AF. Both groups of patients presented HF. All the following assessments and characteristics refer to the non-valvular AF group. The mean age of our studied group (non-valvular AF) was 73.79 years with a SD of 10.487 (p=0.000), as seen in (Table 1), with slight male predominance (54.4% vs. 45.6%).
Demographic data and baseline characteristics are shown in (Table 2). In our study group of non-valvular AF and HF patients, we found 3.8 % first detected AF, 28.9% paroxysmal AF, 17.1% persistent AF, 25.1% long standing persistent AF, and 25.1% permanent AF. A third (86 SD 0.294) of our patients presented HF with preserved ejection fraction (HFpEF), almost a quarter of our study group (64 SD 0.337) were included in HF mid-range ejection fraction (HFmrEF), and the majo-rity have HF with reduces ejection fraction HFrEF.
Between the risk factors found in our study group we specify: hypertension (54.3%), dilative cardiomyo-pathy (47.1%), ischemic heart disease (44.9%), dyslipi-demia (38.0%), chronic obstructive pulmonary disea-se (36.1%), endocrine disorders (6.1%), and valvular heart disease (72.62%), and pacemakers (4.9%). In our study group, 66.2% patients presented at echography mitral regurgitation. A percentage of 31.7% have mild regurgitation, 22.8 % have moderate and 11.8 % have severe mitral regurgitation. Aortic regurgitation was encountered in 20.5% patients, most of them have mild aortic regurgitation (SD 0.291). Tricuspid regur-gitation was noted in 39.5% patients, almost half of theme presented mild tricuspid regurgitation. Aortic stenosis was found in 16.8% patients, and mitral ste-nosis in 4.2% patients. A number of 19 (SD 0.263), patient presented with native heart valve involvement. Pulmonary hypertension was found in 22.8% patients most of them presenting moderate or severe pulmo-nary hypertension.
Comorbidities found among our patients were: anxiety disorders (37.3%), chronic kidney disease (31.2%), diabetes mellitus (28.1%), arthrosis (28.1%), hepatic disorders (25.5%), obesity (24.0%), malignancy (22.5%), left bundle branch (12.2%), Parkinson disea-se (9.9%), hemorrhagic events (8.7%), stroke (8.4%), peripheral vascular disease (7.2%), anemia (6.8%), and right bundle branch (5.3%) All the associated comor-bidities and risk factors are highlighted in (Table 3).
At discharge, in the non-valvular AF group, 50.2% have beta-blockers prescribed, 42.6% angiotensin converting enzyme inhibitors, 23.6% angiotensin II receptor blockers, 31.9% on digoxin, 20.9% on cal-cium antagonists, 81.4% on diuretics, 32.3% on aspirin, 45.6% statins, 29.7% antiarrhythmic agents. More than one third of the patients in our study group have a non-vitamin K antagonist oral anticoagulant (NOAC) prescription: 15.6% used Dabigatran, 14.1% take Api-xaban, and 8.4% are on Rivaroxaban, proving once more the underutilization of NOAC.
In (Figure 1) we emphasize the thromboembolic risk profile estimated throughout the CHA2DS2 – VASC score, whose median in our study was 5.19 SD 1.337 the majority of the cases having a score ≥2. Figure 2 highlights the hemoragic risk profile esti-mated throughout the HAS-BLED score, whose me-dian in our study was 3.08 SD 1.5, the majority of the cases having a score ≥3. In our study group, we had an in-hospital mortality rate of 12.9% (sudden cardiac death were also inclu-ded in these numbers), compared to an in-hospital mortality rate of 24.3% in the other group (valvular AF) (p= 0.02).

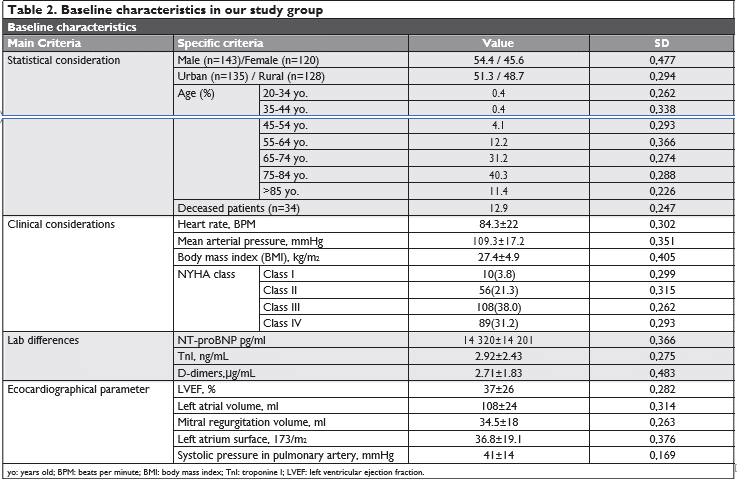
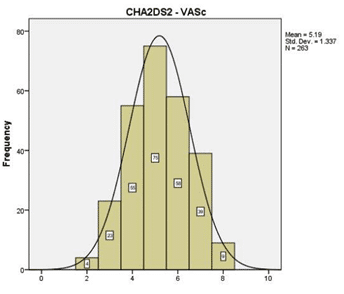
Figure 1. The thromboembolic risk profile estimated in our study group throughout the CHA2DS2-VASC score.
DISCUSSION
This study targets a specific group of population and assess several aspects related to non-valvular AF in patients hospitalized with HF. The results confirm a high prevalence of non-valvular AF in HF in our clinic, with a high thromboembolic risk profile and low rate of use of NOAC. Atrial fi brillation was associated with several comorbidities, implying a high mortality rate. Compared with international registries, the age of our study group, was similar with that reported in the following registries: EORP-AF Pilot survey14 (71.2 years), EHFS (71.3 years)18; ADHERE (72.5 years)19; and almost 5 years younger than in OPTIMIZE-HF trial (78 years)20.
Differences as the subtypes of AF can be noted mainly regarding the first diagnosed AF, which in our study was little encountered (3.8%) compared to other registries like EORP-AF Pilot survey14 (35%). The same observation can be made concerning long standing persistent AF which represented a quarter of our patients, but in the EORP-AF Pilot survey14 it was only found in 5.3% of the patients. These could be ex-plained by the fact that in our study we only presented the non-valvular AF patients, not all AF patients like in other registries. Non-valvular AF was not a pre-speci-fied subgroup in the specified studies.
Only a few studies have assessed the subtype of HF (according to the last classification) in cases of AF. Di-fferences can also be noticed between these types of HF: we found a higher prevalence of HFrEF (43.0%), compared to HFpEF (32.7%) and HFmrEF (24.3%). In the AF registries were HFrEF can be found in a quar-ter of the patients and HFpEF in 45.1%. These facts could be explained due to the emergency profile of our clinic and the limited admission of HF in the car-diology clinic, resulting in more severe and decom-pensated cases and the poorer clinical status of our patients. Clinical trials, such as CHARM study21 state a much higer prevalence of HFpEF than in our study, but these could be explained throughout the introduction of HFmrEF.
We could identify differences betwen our group and other registries concerning some risk factors like hypertension and endocrine disorders. We found hypertension in more than half of our group (54.3%), compared to a percentage of 73.9% in the EORP-AF Pilot survey14, 77% in the ADHERE study22, 52% in the Swedish Heart Failure Registry23 (that included 7.392 pa-tients with HFrEF and AF) and 46% in the AATAC trial24. Smaller published studies25 reported simillar prevalence to ours. The association of the remaining risk factors for AF-HF found in the present study has been reported similarly, in other studies.
Similarly, the association of comorbidities found in our patients has been reported in the international li-terature.
In terms of therapeutical agents used, we could find some diferences between our group and the other re-gistries concerning the prescription of beta-blockers and diuretics. A percentage of 50.2% patients in our study received betablockers compared to 77.4% in the EORP-AF Pilot survey14 78% in the AATAC trial24, and 79% in AF-CHF trial26. This is interesting given that be-ta-blockers are now a standardized part of treatment in AF and FH following numerous randomized clini-cal trials reporting a substantial reduction in all-cause mortality27, cardiovascular death and hospitalization. The undepriscription of beta-blockers could be explained by the frailty of our patients and could refl ect the severity of HF26.
Non-valvular AF was mostly assesd in the cohorts enrolled in trials on NOAC28-31, based on highly-se-lected patients. The European Heart Rhythm Association position paper states that the currently unique con-traindications to NOAC are patients with mechanical heart valves and those with moderate-to-severe mitral stenosis. Patients with native heart valve involvement, regardless of their severity, are suitable for NOAC therapy. Patients with bioprosthetic heart valves and mitral valve repair may be suitable for NOAC except for the fi rst 3-6 months postoperatively. Patients with transaortic valve implantation or percutaneous trans-luminal aortic valvuloplasty are also considered as be-ing eligible for NOAC, but future studies are required to prove the level of evidence for NOAC use, parti-cularly in these patients32. The bleeding risk, for the last population (often requiring a combination with antiplatelet therapy), has to be carefully asseed and it’s ultimately the decision of the physician who assesses the risk and benefit.
Despite the limitations on NOAC usage due to their cost, the proportion of patients treated with NOAC are progressively increasing, proving their effectiveness and safety. In our study NOAC were more commonly prescribed (38.1%) than in other re-ported studies: 7.2% in EORP-AF Pilot survey14, 14.1% in ORBIT-AF33 registry, 23% in GLORIA-AF34 trial. This suggests a much better adherence to evidence and guidlines recommendation, but familiarity with prescribing NOAC may still be a challenge.
There is growing interest on assessing if the trom-bembolic risk, as well as the risk of death, reagarding clinical presentation is related to the type of AF, as investigated by numerous clinical trials35. In our study, the CHA2DS2-VASc score showed an elevated throm-boembolic risk profile: median of 5.19 with a SD of 1.337, and most of the patients have a score ≥2. The results obtained suggest that there is still a underuti-lization of NOAC in these patients, mainly those at higher thromboembolic risk, which might have an im-portant impact on mortality after hospital discharge, as shown in the ADHERE Study22. Underutilization of NOAC in patients with AF and HF has also been re-ported in the literature. Our findings are in concor-dance with specification of the EORP-AF Pilot survey36 which states that estern countries have a tendency in underuntilizaton of NOAC. Stroke and bleeding risks were higher in AF patients with HF.
Probably only the physicians individual experience coroborated with a better knowledge on these direct oral anticoagulants (and on their effect) might help to increase the anticoagulation use rate in these pa-tients. The lack of a heart failure unit prones in selec-ting more severely ill patients to be hospitalized in the general cardiology or internal medicine department. Greater complexity of these cases, requiring a longer length of stay (in hospital) for clinical compensation just underlines the importance of a specialized heart team and unit that could alleviate these patients.
In the present study, in-hospital mortality was 9.26%, greater than that reported in the international literature, such as the ADHERE Registry22 (4%), once again suggesting the more severe profile of the pati-ents in this study. Our numbers obtained are higher, but these could be explained due to the emergency profi le of the hospital and because of the presence of a stroke unit (a high addressability from all the nearby counties).
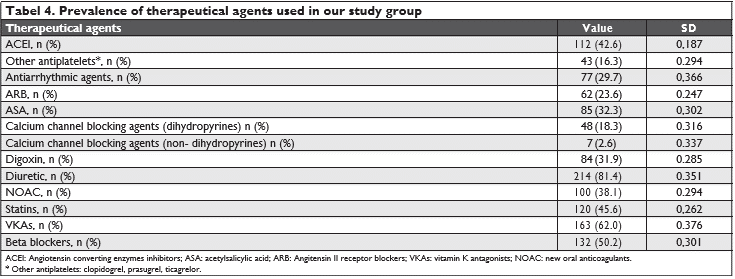
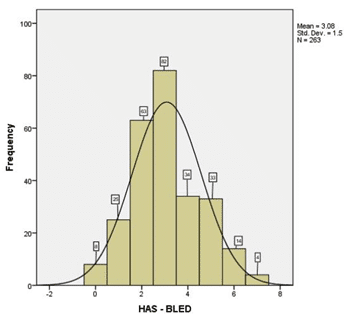
Figure 2. The hemoragic risk profile estimated in our study group throughout the HAS-BLED score.
LIMITATIONS OF THE STUDY
This is a small single-center retrospective non-rando-mized study, without a control group. This makes in-terpretation of data difficult. The data shown in this study represent the clinical practice in our center, so they cannot be generalized. However, compared with the data in the literature the prevalence of AF comor-bidities is quite similar with the general data. We did not perform echocardiographic assessment of the left atrial strain and of the left atrial functions.
CONCLUSION
We found in our study, that the presence of non valvular AF in HF patients is associated with a high number of risk factors, comorbidities and high in-ho-spital mortality. Knowledge of the underlying factors and their management is the cornerstone for optimal treatment in AF patients.
Despite the extensive amount of literature that treats these conditions (HF and AF) individually and combined, there is still a demanding need for further research.
Conflict of interest: none declared
Funding: This research did not receive any specific grant from funding agencies in the public, commercial or not-for profit sectors.
References:
1. P Kirchhof, S Benussi, D Kotecha, A Ahlsson, D Atar, B Casadei, M Castella, HC Diener, H Heidbuchel, J Hendriks, G Hindricks, AS. Manolis, Jonas Oldgren, BA Popescu, U Schotten, B Van Putte, P Vardas, S Agewall, J Camm, G Baron Esquivias, W Budts, S Car-erj, F Casselman, A Coca, R De Caterina, S Deftereos, D Dobrev, JM. Ferro, G Filippatos, D Fitzsimons, B Gorenek, M Guenoun, SH. Hohnloser, P Kolh, GYH. Lip, A Manolis, J McMurray, P Ponikowski, R Rosenhek, F Ruschitzka, I Savelieva, S Sharma, P Suwalski, JL Tama-rgo, CJ. Taylor, IC. Van Gelder, AA. Voors, S Windecker, JL Zamo-rano, K Zeppenfeld; 2016 ESC Guidelines for the management of atrial fibrillation developed in collaboration with EACTS, European Heart Journal, Volume 37, Issue 38, 7 October 2016, Pages 2893– 2962, https://doi.org/10.1093/eurheartj/ehw210
2. Bjorck S, Palaszewski B, Friberg L, Bergfeldt L, Atrial fibrillation, stroke risk, and warfarin therapy revisited: a population-based study, Stroke 2013, 44:3103–3108.
3. Haim M, Hoshen M, Reges O, Rabi Y, Balicer R, Leibowitz M, Pro-spective national study of the prevalence, incidence, management and outcome of a large contemporary cohort of patients with in-cident non-valvular atrial fibrillation, J Am Heart Assoc, 2015, 4:e001486.
4. Chugh SS, Havmoeller R, Narayanan K, Singh D, Rienstra M, Benja-min EJ, Gillum RF, Kim YH, McAnulty JH Jr, Zheng ZJ, Forouzanfar MH, Naghavi M, Mensah GA, Ezzati M, Murray CJ, Worldwide epide-miology of atrial fibrillation: a Global Burden of Disease 2010 Study, Circulation 2014, 129:837–847
5. Andersson T, Magnuson A, Bryngelsson IL, Frobert O, Henriksson KM, Edvardsson N, Poci D, All-cause mortality in 272,186 patients hospitalized with incident atrial fibrillation 1995-2008: a Swedish na-tionwide long-term case-control study, Eur Heart J, 2013,34:1061– 1067
6. Kotecha D, Holmes J, Krum H, Altman DG, Manzano L, Cleland JG, Lip GY, Coats AJ, Andersson B, Kirchhof P, von Lueder TG, We-del H, Rosano G, Shibata MC, Rigby A, Flather MD, Beta-Blockers in Heart Failure Collaborative Group. Efficacy of beta blockers in patients with heart failure plus atrial fibrillation: an individual-patient data meta-analysis, Lancet, 2014,384:2235–2243
7. Wolf PA, Abbott RD, Kannel WB, Atrial fibrillation as an indepen-dent risk factor for stroke: the Framingham Study, Stroke, 1991,22: 983–988
8. Emdin CA, Wong CX, Hsiao AJ, Altman DG, Peters SA, Woodward M, Odutayo AA, Atrial fibrillation as risk factor for cardiovascular disease and death in women compared with men: systematic review and meta-analysis of cohort studies, BMJ 2016, 532:h 7013
9. Ko D, Rahman F, Schnabel RB, Yin X, Benjamin EJ, Christophersen IE, Atrial fibrillation in women: epidemiology, pathophysiology, pre-sentation, and prognosis, Nat Rev Cardiol, 2016,13:321–332
10. L Gigli, P Ameri, G Secco, G De Blasi, R Miceli, A Lorenzoni, F Torre, F Chiarella, C Brunelli, M Canepa Clinical characteristics and prognostic impact of atrial fibrillation in patients with chronic heart failure,World J Cardiol 2016 November 26; 8(11): 647-656 ISSN 1949-8462 (online) DOI: 10.4330/wjc.v8.i11.647
11. Mozaffarian D, Benjamin EJ, Go AS, et al. Heart disease and stroke statistics–2015 update: a report from the American Heart Associa-tion. Circulation 2015;131:e29-322. PUBMED | CROSSREF
12. SA Batul, R Gopinathannair, Atrial Fibrillation in Heart Fail-ure: a Therapeutic Challenge of Our Times, Korean Circ J. 2017 Sep;47(5):644-662, https://doi.org/10.4070/kcj.2017.0040, pISSN 1738-5520·eISSN 1738-5555]
13. Eggimann L, Blum S, Aeschbacher S, Reusser A, Ammann P, Erne P, et al. (2018) Risk factors for heart failure hospitalizations among pa-tients with atrial fibrillation. PLoS ONE 13(2): e0191736. https://doi. org/10.1371/journal. pone.0191736
14. Lip GY, Laroche C, Popescu MI, Rasmussen LH, Vitali-Serdoz L, Dan GA, Kalarus Z, Crijns HJ, Oliveira MM, Tavazzi L, Maggioni AP, Bo-riani G, Heart failure in patients with atrial fi brillation in Europe: a report from the EURObservational Research Programme Pilot sur-vey on Atrial Fibrillation, Eur J Heart Fail. 2015 Jun;17(6):570-82. doi: 10.1002/ejhf.254. Epub 2015 Mar 2.]
15. Pathak RK, Middeldorp ME, Lau DH, Mehta AB, Mahajan R, Twomey D, Alasady M, Hanley L, Antic NA, McEvoy RD, Kalman JM, Ab-hayaratna WP, Sanders P, Aggressive risk factor reduction study for atrial fibrillation and implications for the outcome of ablation: the ARREST-AF cohort study, J Am Coll Cardiol, 2014, 64:2222–2231
16. Abed HS, Wittert GA, Leong DP, Shirazi MG, Bahrami B, Middel-dorp ME, Lorimer MF, Lau DH, Antic NA, Brooks AG, Abhayaratna WP, Kalman JM, Sanders P, Effect of weight reduction and cardio-metabolic risk factor management on symptom burden and severity in patients with atrial fibrillation: a randomized clinical trial, JAMA, 2013, 310:2050–2060
17. Kirchhof P, The future of atrial fibrillation management: integrated care and stratified therapy, Lancet, 2017 Oct 21, 390(10105):1873-1887, doi:10.1016/S0140-6736(17)31072-3, Epub 2017 Apr 28.
18. Cleland JG, Swedberg K, Follath F, Komajda M, Cohen-Solal A, Agui-lar JC, et al; Study Group on Diagnosis of the Working Group on Heart Failure of the European Society of Cardiology. The EuroHeart Failure survey programme– a survey on the quality of care among patients with heart failure in Europe. Part 1: patient characteristics and diagnosis. Eur Heart J. 2003;24(5):442-63.
19. Gheorghiade M, Filippatos G. Reassessing treatment of acute heart failure syndromes: the ADHERE Registry. Eur Heart J Suppl. 2005;7(Suppl B):B13-B19.
20. Hernandez AF, Hammill BG, O’Connor CM, Schulman KA, Curtis LH, Fonarow GC. Clinical effectiveness of beta-blockers in heart failure: findings from the OPTIMIZE-HF (Organized Program to Initi-ate Lifesaving Treatment in Hospitalized Patients with Heart Failure) Registry. J Am Coll Cardiol. 2009;53(2):184-92Grau AJ, Weimar C, Buggle F, Heinrich A, Goertler M, Neumaier S, Glahn J, Brandt T, Hacke W, Diener H, Risk factors, outcome, and treatment in sub-types of ischemic stroke:the German stroke data bank, Stroke 2001, 32:2559–2566
21. Olsson LG, Swedberg K, Ducharme A, Granger CB, Michelson EL, McMurray JJ, et al; CHARM Investigators. Atrial fibrillation and risk of clinical events in chronic heart failure with and without left ventricu-lar systolic dysfunction: results from the Candesartan in Heart fail-ure-Assessment of Reduction in Mortality and morbidity (CHARM) program. J Am Coll Cardiol. 2006;47(10):1997-2004Chien K, Su T, Hsu H, Chang W, Chen P, Chen M, Lee Y, Atrial fibrillation preva-lence, incidence and risk of stroke and all-cause death among Chi-nese, Intern J Cardiol, 2010, 139:173-180
22. Yancy CW, Lopatin M, Stevenson LW, De MT, Fonarow GC. Clini-cal presentation, management, and in-hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database, J Am Coll Cardiol , 2006, vol. 47 (pg. 76-84)Nieuwlaat R, Olsson SB, Lip GY, Camm AJ, Breithardt G, Capucci A, Meeder JG, Prins MH, Levy S, Crijns HJ, Euro Heart Survey Investigators, Guideline-adherent antithrombot-ic treatment is associated with improved outcomes compared with undertreatment in high-risk patients with atrial fi brillation. The Euro Heart Survey on Atrial Fibrillation, Am Heart J, 2007, 153:1006– 1012
23. Lund LH, Benson L, Dahlstrom U, Edner M. Association between use of renin-angiotensin system antagonists and mortality in patients with heart failure and preserved ejection fraction, JAMA , 2012, vol. 308 (pg. 2108-2117)
24. L Di Biase, P Mohanty, S Mohanty, P Santangeli, C Trivedi, D Lak-kireddy, M Reddy, P Jais, S Themistoclakis, A Dello Russo, M Ca-sella, G Pelargonio, ML Narducci, RSchweikert, P Neuzil, J Sanchez, R Horton, S Beheiry, R Hongo, S Hao, A Rossillo, G Forleo, C Ton-do, J. D Burkhardt, M Haissaguerre, A Natale, Ablation vs. Amioda-rone for Treatment of Persistent Atrial Fibrillation in Patients With Congestive Heart Failure and an Implanted Device: Results From the AATAC Multicenter Randomized Trial, Circulation. 2016;CIR-CULATIONAHA.115.019406, originally published March 30, 2016, https://doi.org/10.1161/CIRCULATIONAHA.115.019406 Hibbard JH, Greene J, What the evidence shows about patient activation: better health outcomes and care experiences; fewer data on costs, Health Aff (Millwood) 2013;32:207–214
25. Lam C.S.P., Rienstra M., Tay W.T., et al. (2017) Atrial fi brillation in heart failure with preserved ejection fraction: association with ex-ercise capacity, left ventricular filling pressures, natriuretic peptides, and left atrial volume. J Am Coll Cardiol HF 5:92–98]
26. J. Cadrin-Tourigny, A. Shohoudi, D. Roy, M. Talajic, R Tadros, B. Mondésert, K Dyrda, L Rivard, JG. Andrade, L Macle, PeG. Guerra, B Thibault, M Dubuc, P Khairy, Decreased Mortality With Beta-Block-ers in Patients With Heart Failure and Coexisting Atrial Fibrillation: An AF-CHF Substudy JACC: Heart Failure, Volume 5, Issue 2, Febru-ary 2017, Pages 99-106, https://doi.org/10.1016/j.jchf.2016.10.015
27. Kotecha D, Piccini JP. Atrial fibrillation in heart failure: what should we do? European Heart Journal. 2015;36(46):3250-3257. doi:10.1093/eurheartj/ehv513.]
28. Lip GY, Frison L, Grind M, SPORTIF Investigators. Stroke event rates in anticoagulated patients with paroxysmal atrial fibrillation. J Intern Med 2008;264:50–61 Google ScholarCrossRefPubMed
29. Al-Khatib SM, Thomas L, Wallentin L, Lopes RD, Gersh B, Gar-cia Det al. Outcomes of apixaban vs. warfarin by type and duration of atrial fi brillation: results from the ARISTOTLE trial. Eur Heart J 2013;34:2464–71.Google ScholarCrossRefPubMed
30. Vanassche T, Lauw MN, Eikelboom JW, Healey JS, Hart RG, Alings M et al. Risk of ischaemic stroke according to pattern of atrial fi-brillation: analysis of 6563 aspirin-treated patients in ACTIVE-A and AVERROES. Eur Heart J 2015;36:281–8. Google ScholarCrossRef-PubMed
31. Steinberg BA, Hellkamp AS, Lokhnygina Y, Patel MR, Breithardt G, Hankey GJet al. Higher risk of death and stroke in patients with persistent vs. paroxysmal atrial fibrillation: results from the ROCK-ET-AF trial. Eur Heart J 2015;36:288–96. Google ScholarCrossRef-PubMed
32. RP Martins, V. Galand, E. Colette, N.Behar, D. Pavin, C Leclercq, JC Daubert, P. Mabo, Defining nonvalvular atrial fi brillation: A quest for clarifi cation, American Heart Journal ,2016, Volume 178 , 161 – 167, https://doi.org/10.1016/j.ahj.2016.05.014]
33. O’Brien EC, Kim S, Thomas L, et al. Clinical Characteristics, Oral Anticoagulation Patterns, and Outcomes of Medicaid Patients With Atrial Fibrillation: Insights From the Outcomes Registry for Bet-ter Informed Treatment of Atrial Fibrillation (ORBIT-AF I) Reg-istry. Journal of the American Heart Association: Cardiovascu-lar and Cerebrovascular Disease. 2016;5(5):e002721. doi:10.1161/ JAHA.115.002721.]
34. SJ. Dubner, MV. Huisman, H-C Diener, J. Halperin, KJ. Rothman, C-S Ma, K. Zint, L. Riou Franca, S. Lu, C. Teutsch, M. Paquette, GYH Lip; Baseline characteristics of patients with atrial fibrillation with and without comorbid heart failure: the GLORIA-AF registry, EP Euro-pace, Volume 19, Issue suppl_3, 1 June 2017, Pages iii39, https://doi. org/10.1093/ehjci/eux141.009
35. G Boriani, C Laroche, I Diemberger, E Fantecchi, MI Popescu, Lars Hvilsted Rasmussen, Gheorghe-Andrei Dan, Zbigniew Kalarus, Lu-igi Tavazzi, Aldo P. Maggioni, Gregory Y.H. Lip; ‘Real-world’ man-agement and outcomes of patients with paroxysmal vs. non-parox-ysmal atrial fibrillation in Europe: the EURObservational Research Programme–Atrial Fibrillation (EORP-AF) General Pilot Registry, EP Europace, Volume 18, Issue 5, 1 May 2016, Pages 648–657, https:// doi.org/10.1093/europace/euv390
36. Lip GY, Laroche C, Dan GA, Santini M, Kalarus Z, Rasmussen LH, Ioachim PM, Tica O, Boriani G, Cimaglia P, Diemberger I, Hellum CF, Mortensen B, Maggioni AP, ‘Real-world’ antithrombotic treat-ment in atrial fibrillation: The EORP-AF pilot survey, Am J Med. 2014 Jun;127(6):519-29.e1. doi: 10.1016/j.amjmed.2013.12.022. Epub 2014 Jan 28.].
 This work is licensed under a
This work is licensed under a