Andreea Calin1,2,*, Anca D. Mateescu1,2,*, Monica Rosca1,2, Carmen Beladan1,2, Roxana Enache1,2, Maria Magdalena Gurzun1, Cosmin Calin1,2, Simona Botezatu1,2, Carmen Ginghina1,2, Bogdan A. Popescu1,2
1 “Carol Davila” University of Medicine and Pharmacy, Euroecolab, Bucharest, Romania
2 “Prof. Dr. C. C. Iliescu” Institute of Cardiovascular Diseases Bucharest, Romania
* These authors contributed equally to this work
Abstract: Aims – We studied the clinical and echocardiographic characteristics of patients (pts) with severe aortic stenosis (AS) and preserved left ventricular ejection fraction (LVEF), according to the flow-gradient classification. Methods and results – We enrolled 165 pts (66±11 years, 95 men) with aortic valve area (AVA) <1 cm2=”” indexed=”” ava=”” 0=”” 6=”” cm2=”” m2=”” br=””> and preserved LVEF (62±6%). Patients were stratifi ed by LV stroke volume index (<35 ml=”” m2=”” low=”” fl=”” ow=”” lf=”” and=”” 35=”” ml=”” br=””> m2 [normal fl ow, NF]) and mean aortic gradient (<40 mmhg=”” low=”” gradient=”” lg=”” and=”” 40=”” mmhg=”” high=”” gradient=”” hg=”” into=”” 4=”” br=””> groups: NF/HG, NF/LG, LF/HG, and LF/LG. LV global longitudinal strain (GLS) was measured in 133 pts. Paradoxical LF/LG AS was present in 4 pts (2.4%). Most pts with LG severe AS had a normal fl ow (18.2%). Compared to NF/HG (121 pts, 73.3%), NF/LG pts had a lower NYHA class and serum BNP, larger AVAs, lower LV mass index, relative wall thickness and smaller LV volumes when compared to NF/HG pts. Worse parameters of LV longitudinal and diastolic function were found in NF/HG pts compared to NF/LG pts: lower GLS (p<0.001), larger left atrial volumes (p=0.03) and higher E/e’ ratios (p=0.009). Conclusion – We found a low prevalence of paradoxical LF/LG severe AS in pts with severe AS and preserved LVEF. According to the proposed criteria, most pts with LG severe AS have a normal transvalvular flow. These pts have a better clinical and echocardiographic profile when compared to pts with NF/HG severe AS. Keywords: aortic stenosis, echocardiography, left ventricular function, global longitudinal strain
INTRODUCTION
Calcifi c aortic valve stenosis (AS) is presently the most common valvular heart disease and a significant health problem1. In the absence of other indications for heart surgery, only patients with severe AS have an indication of aortic valve replacement2,3. Therefore, an accurate grading of AS is mandatory for each patient. The currently used echocardiographic severity criteria are
based on the measurement of peak transvalvular velocity, mean gradient and calculation of aortic valve area (AVA) by continuity equation. Conditions that reflect the discordance in currently used AS severity criteria are increasingly important in clinical practice. The most frequent discordant AS grading pattern is low gradient (LG) severe AS with preserved left ventricular ejection
fraction (LVEF), these patients presenting with an indexed AVA ≤0.6 cm2/m2, a mean aortic gradient <40 mm Hg and an LVEF >50%. Patients with a low transvalvular flow (defined by a stroke volume index (SVi) <35 ml/m2) are classified as having a paradoxical low flow (LF) low gradient severe AS4. Discordant echocardiographic grading of AS severity in a patient with normal transvalvular flow may be related to multiple factors, such as measurement errors5, small body size, reduced arterial compliance6,7, or discordance between AVA and mean aortic gradient cutpoints used for the definition of severe AS8. Minners et al. have showed that from a hemodynamic standpoint a mean aortic gradient of 40 mmHg does not correspond to an AVA of 1.0 cm2, but rather to an AVA of 0.8 cm2.8 Therefore, some authors have proposed changing the criteria currently used to defi ne severe AS, namely lowering the AVA cutpoint from 1 to 0.8 cm2.9
The prognosis and management of patients with LG severe AS is not clear so far. In order to better characterize this subset of AS patients, we studied the clinical and echocardiographic characteristics of patients with severe AS and preserved LVEF, according to the recently proposed fl ow-gradient classification4.
METHODS
We prospectively enrolled 165 consecutive patients with severe AS (AVA <1 cm2, indexed AVA <0.6 cm2/ m2) and preserved LVEF (>50%) who were referred to our echo lab. We excluded patients with atrial fibrillation, more than mild aortic or mitral regurgitation, mitral stenosis/prosthesis, and patients with coronary artery disease (by history, echocardiography, or coronary
angiography). All patients gave their informed consent to participate in the study, the protocol of which was approved by our local Ethics Committee. All patients were subject to a comprehensive echocardiography (2D, conventional and tissue Doppler, 2D strain using speckle tracking echocardiography). Images were obtained using a commercially available cardiac ultrasound machine (Vivid 7 Dimension and Vivid E9, GE Healthcare, Horten, Norway) equipped with a 4S probe. Standard views and techniques were used according to the American Society of Echocardiography/
European Association of Echocardiography recommendations10. Left ventricular volumes and LVEF were measured using the modifi ed Simpson’s rule from apical four- and two-chamber views and were normalized to body surface area (BSA). LV mass was calculated by the equation of Devereux11 and indexed to BSA. The degree of calcifi cation of the aortic valve was assessed using the classifi cation proposed by Rosenhek et al.: 1, no calcification; 2, mildly calcifi ed (small isolated spots); 3, moderately calcified (multiple larger spots); and 4, heavily calcified (extensive thickening and calcification of all cusps)12.
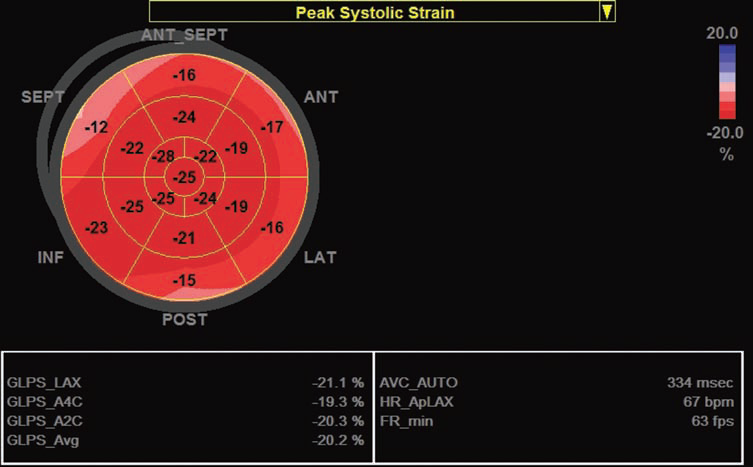
Figure 1. The illustration of LV longitudinal deformation measured off-line in 17 segments using a 2D strain software in a patient with severe aortic stenosis. Global longitudinal strain is within normal range in this patient (-20%).
Continuous-wave Doppler was used to measure the aortic transvalvular maximal velocities; peak and mean gradients were calculated using the simplified Bernoulli equation. LV stroke volume was calculated using pulsed wave Doppler as follows: LV outflow tract area×LV outfl ow tract velocity-time integral (measured by PW Doppler). Aortic valve area was calculated using the continuity equation13. Peak systolic (S) and peak early diastolic (e’) mitral annular velocities were obtained by pulse-wave tissue Doppler imaging from the apical four-chamber view using both the septal and the lateral sites. The average e’ was used to calculate the ratio of peak early-diastolic transmitral flow velocity E/e’ in order to estimate LV fi lling pressures14. Global longitudinal LV strain was measured by speckle tracking echocardiography in a 17 segments model (Figure 1) using 2D images acquired with a frame rate between 50-70 fps in the apical views centered on the
LV. This was feasible in 133 pts with an adequate acoustic window.
STATISTICAL ANALYSIS
Patients were stratified according to LV stroke volume index (<35 ml/m2 [LF] vs ≥35 ml/m2 [NF]) and aortic gradient (<40 mm Hg [LG]vs ≥40 mm Hg [high gradient, HG]) into 4 groups according to the flow-gradient classification4: group 1, LF/HG; group 2, LF/LG; group 3, NF/LG; or group 4, NF/HG. Data are reported as mean±SD or number (percentage). Skewed data such
as BNP serum values were logarithmically transformed and logBNP values were used in comparative analyses. Student t tests were used to compare continuous variables, and Pearson 2 or Fisher exact tests were used to compare categorical variables between groups.
RESULTS
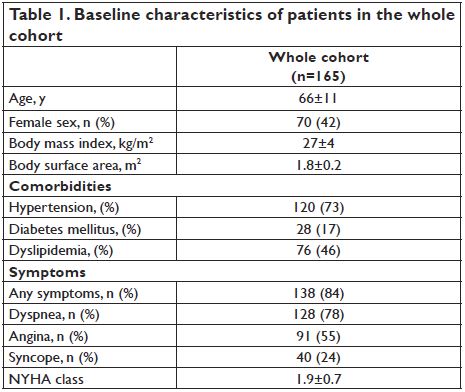
A total of 165 patients (66±11 yrs, 57.6% male) were included in our study. Baseline characteristics of patients in the whole cohort are presented in Table 1. The mean AVA was 0.7±0.2 cm2, AVAi 0.4±0.1 cm2/m2, mean aortic gradient 58.2±20.3 mm Hg, and peak transvalvular velocity 4.8±0.8 m/s. Low gradient AS was present in 34 pts (21%) of our study group. Paradoxical LF/LG
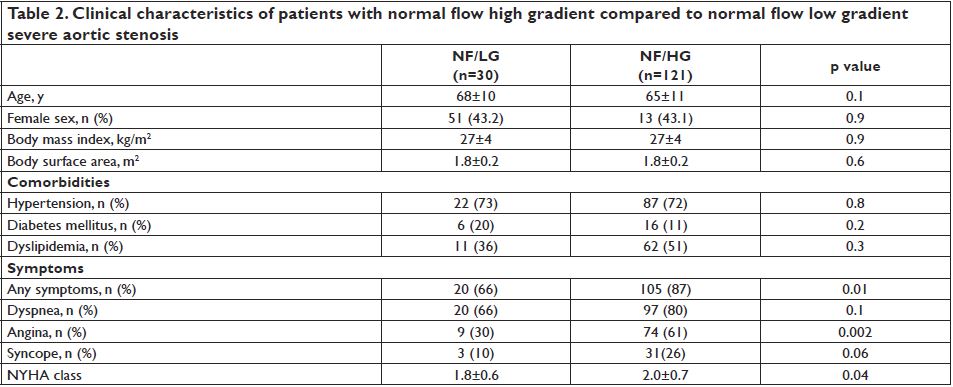
severe AS was present in 4 pts (mean age 67±5 yrs, three female patients, all of them symptomatic), while most patients with LG severe AS had a normal transvalvular flow (30 pts, 18.2%). The majority of pts were classified as NF/HG (121 pts, 73.3%), while 10 pts (6% of the study group) had LF/HG AS. Most patients were symptomatic (84%). However, compared to NF/HG
patients, those having NF/LG were less symptomatic, with a lower prevalence of angina and syncope and a lower NYHA class.
The clinical characteristics of these two groups are shown in Table 2. The serum BNP values were available in 76 patients – range 11-4059 pg/ml, median value 207 pg/ml (86-334). Patients with NF/HG had significantly higher BNP values compared to NF/LG patients (p=0.03).
ECHOCARDIOGRAPHIC CHARACTERISTICS
Both AVA and indexed AVA were higher in patients included in the NF/LG group when compared to patients with NF/HG-AS (0.86±0.10 cm2 vs. 0.69±0.16 cm2, p<0.001 and 0.47±0.06 cm2/m2 vs. 0.38±0.08 cm2/ m2, p<0.001). There was a trend toward a higher level of calcification of the aortic valve in patients with NF/HG-AS compared to patients in the NF/LG group
(p=0.06). Blood pressure values at the time of the echocardiographic examination were not different between groups. Valvulo-arterial impedance was lower in patients with NF/LG-AS compared to NF/HG pts (3.9±0.6 vs. 4.3±0.8 mmHg/ml/m2, p=0.03). Patients with NF/ LG-AS also had a lower LV stroke volume index (43±4 vs. 47±7 ml/m2, p=0.001) and smaller LV outflow tract
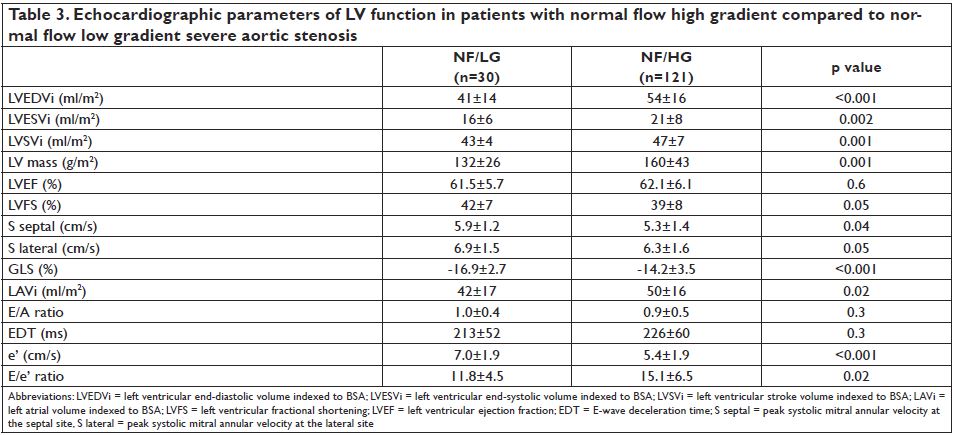
diameters (20.4±1.6 vs. 21.2±2.1 mm, p=0.04). The echocardiographic parameters of LV systolic and diastolic function in patients with NF/LG -AS and NF/HG-AS are presented in Table 3. Patients with NF/ LG had lower indexed LV mass (p=0.002) and smaller LV volumes (p<0.004) when compared to NF/HG pts. Although LVEF was similar between groups (62±6 vs. 61±6%, p=0.6), worse parameters of LV longitudinal and diastolic function were found in NF/HG compared to NF/LG patients. Compared to patients with NF/LGAS, lower systolic myocardial velocities and a more impaired global longitudinal deformation were found in patients with NF/HG-AS, revealing a poorer LV systolic function.These patients had also larger indexed left atrial volumes (p=0.02) and higher E/e’ ratios (p=0.03) indicating a higher degree of LV diastolic dysfunction.
DISCUSSION
In the present study, we found that most patients with low gradient severe AS have a normal transvalvular flow when classified according to the flow-gradient classification. Compared to patients with the most frequent type of severe AS (NF/HG-AS) these patients have a lower degree of LV afterload and a better LV function. This is the first study that shows worse LV longitudinal deformation in NF/HG patients with AS compared to patients with NF/LG-AS. This information adds to the previously published data indicating a better prognosis in patients with NF/LG-AS15. Similar to the data published by Eleid et al.15, using the proposed cut-off value of 35 ml/m2 for the definition of LF, we found a very low prevalence of paradoxical LF/LG -AS (<3%). Left ventricular stroke volume is an important determinant of the transvalvular aortic gradient. It was suggested that in patients with LG severe AS, stroke volume might be decreased as a result of the small body habitus, concentric remodeling with reduced ventricular cavity size, and higher arterial impedance4. Both body surface area and body mass index are similar between different groups of AS patients in our study, although patients with NF/LG-AS have smaller LV volumes that may explain the low transvalvular gradient. In our study, patients with NF/LG-AS had larger AVA
and indexed AVA compared to other groups, although the values were below the cut-off that is currently used by the guidelines to defi ne severe AS2. Of note, LV outfl ow tract diameters in patients with NF/LG-AS were smaller than in NF/HG patients, and this is a key element in the AVA calculation. A study by Michelena et al.16 showed that in patients with LV outfl ow tract
diameters ≤2.2 cm, the majority of patients with AVA <1cm2 have a mean gradient below 40 mm Hg. This is important for the illustration of the discordance that exists in currently used AS severity criteria. The prognosis of patients with low gradient AS and their management are still controversial17. Our results demonstrate that these patients have a lesser degree of LV hypertrophy, a milder impairment of LV longitudinal deformation and lower E/e’ ratio suggesting lower LV filling pressures. These data suggest that patients with NF/LG AS may, in fact, have moderate AS with a milder degree of LV dysfunction. Given the lower prevalence of symptoms in this subset of patients, we suggest that a lower AVA cut-off value should be used to define severe AS.
STUDY LIMITATIONS
The observational character and the relatively small sample size, compared to that of the previously published data, are inherent limitations of the present study. However, this investigation represents the first study that shows more impaired LV longitudinal deformation in NF/HG patients with AS and preserved LVEF compared to NF/LG-AS patients, adding new data with
clinical implications to the previously published research. Nevertheless, it is likely that the prevalence of LF/ LG-AS patients was underestimated in our study since we have excluded patients with atrial fibrillation and/ or associated mitral valve disease, although our results are similar to that reported by Eleid et al in a larger series15.
CONCLUSION
In a relatively large number of patients with severe isolated AS and preserved LVEF, we found a very low prevalence of paradoxical low fl ow low gradient AS. According to the proposed flow-gradient criteria, most patients with low gradient severe AS have a normal transvalvular fl ow. These patients have a better clinical and echocardiographic profi le when compared to patients with high gradient severe AS. These fi ndings have potential implications for the assessment of AS severity and consequent management.
Acknowledgements: This work was supported by a grant of the Romanian Ministry of National Education, CNCS – UEFISCDI, project number PN-II-IDPCE- 2012-4-0560 (contract 21/2013) and by the Sectoral Operational Programme Human Resources Development (SOP HRD), financed from the European Social Fund and by the Romanian Government under the contract number POSDRU/159/1.5/S/132395. We gratefully acknowledge the support of our colleagues who have referred patients for this study.
References
1. Iung B, Baron G, Butchart EG, Delahaye F, Gohlke-Bärwolf C, Levang OW, Tornos P, Vanoverschelde JL, Vermeer F, Boersma E, Ravaud P, Vahanian A. A prospective survey of patients with valvular heart disease in Europe: the Euro Heart Survey on valvular heart disease. Eur Heart J 2003; 24: 1231–1243.
2. Vahanian A, Alfi eri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schäfers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M. Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur Heart J 2012;33: 2451–2496.
3. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Guyton RA, O’Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM 3rd, Thomas JD. 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J Am CollCardiol 2014;63:2438-2488.
4. Dumesnil JG, Pibarot P, Carabello B. Paradoxical low fl ow and/or low gradient severe aortic stenosis despite preserved left ventricular ejection fraction: implications for diagnosis and treatment. Eur Heart J 2010;31:281–289.
5. Baumgartner H, Hung J, Bermejo J, Chambers JB, Evangelista A, Griffin BP, Iung B, Otto CM, Pellikka PA, Quiñones M. Echocardiographic assessment of valve stenosis: EAE/ASE recommendations for clinical practice. J Am SocEchocardiogr 2009;22:1–23.
6. Clavel MA, Messika-Zeitoun D, Pibarot P, Aggarwal SR, Malouf J, Araoz PA, Michelena HI, Cueff C, Larose E, Capoulade R, Vahanian A, Enriquez-Sarano M. The complex nature of discordant severe calcified aortic valve disease grading: new insights from combined Doppler-echocardiographic and computed tomographic study. J Am Coll Cardiol 2013;62:2329–38.
7. Kadem L, Dumesnil JG, Rieu R, Durand LG, Garcia D, Pibarot P. Impact of systemic hypertension on the assessment of aortic stenosis. Heart 2005;91:354–61.
8. Minners J, Allgeier M, Gohlke-Baerwolf C, Kienzle RP, Neumann FJ, Jander N. Inconsistent grading of aortic valve stenosis by current guidelines: haemodynamic studies in patients withapparently normal left ventricular function. Heart 2010;96:1463–8.
9. Zoghbi WA. Low-gradient “severe” aortic stenosis with normal systolic function: time to refi ne the guidelines? Circulation 2011;123:838– 40.
10. Lang RM, Badano LP, Mor-Avi V, Afi lalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging 2015;16:233-270.
11. Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I et al. Echocardiographicassessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol 1986;57:450–8.
12. Rosenhek R, Binder T, Porenta G, Lang I, Christ G, Schemper M, Maurer G, Baumgartner H. Predictors of outcome in severe, asymptomatic aortic stenosis. N Engl J Med 2000;343:611-7.
13. Zoghbi WA, Farmer KL, Soto JG, Nelson JG, Quinones MA. Accurate noninvasive quantification of stenotic aortic valve area by Doppler echocardiography. Circulation 1986;73:452–9.
14. Ommen SR, Nishimura RA, Appleton CP, Miller FA, Oh JK, Redfield MM, Tajik AJ. Clinical utility of Doppler echocardiography and tissue Doppler imaging in the estimation of left ventricular fi lling pressures: a comparative simultaneous Doppler-catheterization study. Circulation 2000;102:1788-94.
15. Eleid MF, Sorajja P, Michelena HI, Malouf JF, Scott CG, Pellikka PA. Flow-gradient patterns in severe aortic stenosis with preserved ejection fraction: clinical characteristics and predictors of survival. Circulation 2013;128:1781-9.
16. Michelena HI, Margaryan E, Miller FA, Eleid M, Maalouf J, Suri R, Messika-Zeitoun D, Pellikka PA, Enriquez-Sarano M.Inconsistent echocardiographic grading of aortic stenosis: is the left ventricular outflow tract important? Heart 2013;99:921-931.
17. Jander N, Minners J, Holme I, Gerdts E, Boman K, Brudi P, Chambers JB, Egstrup K, Kesäniemi YA, Malbecq W, Nienaber CA, Ray S, Rossebø A, Pedersen TR, Skjærpe T, Willenheimer R, Wachtell K, Neumann FJ, Gohlke-Bärwolf C. Outcome of patients with low-gradient “severe” aortic stenosis and preserved ejection fraction. Circulation 2011;123:887-895.
 This work is licensed under a
This work is licensed under a