Daniela-Noela Radu1, Dan M. Dorobantu1, Roxana Enache1,2
1 „Prof. Dr. C. C. Iliescu” Emergency Institute of Cardiovascular Diseases, Bucharest, Romania
2 „Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania
Abstract: Aims – Fixed subaortic stenosis (SAS) can be classified into a complex form when it is associated with other defects involving the left ventricular (LV) infl ow and outfl ow tract and a simple form when this is not the case. Previous stu-dies have suggested that these two forms have different outcomes and might represent different pathologies altogether. Our purpose was to study the characteristics and outcomes of operated and unoperated complex SAS and simple SAS patients in a single tertiary centre of general cardiology. Methods – A total of 93 consecutive patients were retrospectively identified between 2003 and 2016. Propensity score matching was used to obtain comparable subgroups of complex SAS (n=24) and simple SAS (n=20) in terms of age (mean 26 years), gender (52% female), functional class (class III 9%, class II 75%) and LV ejection fraction (mean 61%) at diagnosis. Results – LV diastolic diameter (51 vs. 47 mm, p=0.08) and interventricular septum thickness (12 vs. 10 mm, p=0.08) seemed to have higher values in the complex SAS group, despite a similar mean aortic gradient in the two subgroups (40 vs. 45 mmHg, p=0.23). There was no significant difference regarding LV ejection fraction (60% vs. 61%, p=0.4). There were more patients with moderate to severe mitral regurgitation in the complex SAS group (25% vs. 10%), whereas moderate to severe aortic regurgitation was equally noticed in both groups (54% vs. 55%). There were more patients who received surgical treatment (54% vs. 25%, p=0.05), with a higher usage of aortic valve replacement in the complex SAS group (38% vs. 0%, p=0.15). Mortality seemed higher in the complex SAS group when compared to simple SAS group (16% vs. 10%, p=0.4), both in operated (15% vs. 0%, p=0.5) and unoperated patients (18% vs. 13%, p=0.6), without reaching the statistical significance due to small sample size. Mean subaortic gradient correlated significantly with left ventricular end-diastolic diameter (negative correlation, R2=0.22, p=0.04), interventricular thickness (positive correlation, R2=0.22, p=0.03) and posterior wall thickness (positive correlation, R2=0.25, p=0.02) in the complex SAS group, with no significant correlations in the simple SAS group. Conclusion – Complex form of subaortic stenosis is associated with a more important left ventricular remodelling probably due to the role played by associated cardiac defects and seems to have a less favourable outcome, both in surgically managed and conservatively treated patients, independent of NYHA functional class and left ventricular ejection fraction at initial diagnosis, with the limitation of the small dimension of the studied group. Further larger and prospective studies are needed in order to confirm these aspects.
Keywords: left ventricular outflow tract obstruction; fixed subaortic stenosis; subaortic membrane
Rezumat: Obiectiv – Stenoza subaortică (SSA) poate fi clasificată într-o formă complexă, când este asociată cu alte defecte ale tractului de intrare sau ieşire al ventriculului stâng (VS) şi o formă simplă, când nu se asociază cu niciun defect de acest tip. Studii realizate anterior au sugerat faptul că aceste două forme au prognostic diferit şi ar putea reprezenta patologii pe deplin diferite. Scopul nostru a fost studierea caracteristicilor şi evoluţiei pacienţilor cu SSA simplă sau SSA complexă, operaţi sau neoperaţi, într-un singur centru terţiar de cardiologie. Metode – Un total de 93 de pacienţi consecutivi au fost identificaţi retrospectiv în perioada 2003-2016. Scoruri de propensitate au fost utilizate pentru a obţine subgrupuri compa-rabile de pacienţi cu SSA complexă (n=24) şi SSA simplă (n=20) din punct de vedere al vârstei (medie 26 de ani), sexului (52% feminin), clasei funcţionale (clasa III 9%, clasa II 75%) şi fracţiei de ejecţie a VS (medie 61%) la diagnostic. Rezultate – Diame-trul diastolic al VS (51 vs 47 mm, p=0,08) şi grosimea septului interventricular (12 vs 10 mm, p=0,08) par a avea valori mai crescute în grupul SSA complexă, în pofida unui gradient aortic mediu similar între cele două subgrupuri (40 vs 45 mmHg, p=0,23). Nu s-a înregistrat o diferenţă semnificativă a fracţiei de ejecţie a VS (60% vs 61%, p=0,4). Stenoza mitrală moderată până la severă a fost mai frecvent întâlnită la pacienţii din grupul SSA complexă (25% vs 10%), în timp ce regurgitarea aortică moderată spre severă a avut o incidenţă egală în ambele subgrupuri (54% vs 55%). S-a constatat un număr mai mare de pacienţi operaţi (54% vs 25%, p=0,05) în grupul SSA complexă, cu înlocuirea valvei aortice în 38% din cazuri. Mortalitatea pare să fie mai mare în cadrul subgrupului cu SSA complexă comparativ cu cel cu SSA simplă (16% vs 10%, p=0,4), atât în cazul pacienţilor operaţi (15% vs 0%, p=0.5), cât şi neoperaţi (18% vs 13%, p=0,6), fără a se atinge o semnificaţie statistică, cel mai probabil din cauza numărului mic de pacienţi. Gradientul subaortic mediu s-a corelat semnifi cativ cu diametrul tele-diastolic al VS (corelaţie negativă, R2=0,22, p=0,04), grosimea septului interventricular (corelaţie pozitivă, R2=0,22, p=0,03) şi grosimea peretelui posterior al VS (corelaţie pozitivă, R2=0,25, p=0,02) în subgrupul SSA complexă, fără nicio corelaţie semnificativă în subgrupul SSA simplă. Concluzii – Stenoza subaortică formă complexă este asociată cu un grad mai mare de remodelare a VS, probabil din cauza rolului jucat de către defectele congenitale asociate şi pare să aibă un prognostic mai nefavorabil, atât la pacienţii operaţi, cât şi la cei neoperaţi, independent de clasa funcţională şi fracţia de ejecţie a VS la diagnosticul iniţial, cu limitarea adusă de dimensiunea mică a lotului studiat. Studii suplimentare prospective sunt necesare pentru a confi rma aceste aspecte.
Cuvinte cheie: obstrucţie a tractului de ejecţie al ventriculului stâng, stenoză subaortică fixă, membrană subaortică
INTRODUCTION
Fixed subaortic stenosis (SAS) is a polymorphic con-dition representing approximately 3-5% of the conge-nital cardiac defects.1 Initially considered a particular form of obstruction of the left ventricle outfl ow tract (LVOT) which appears mostly in the pediatric popu-lation, we know nowadays that this pathology also affects adults, with a different clinical course between the two groups, slower in the adult population. The most frequent morphological phenotype is discrete membranous SAS, consisting of a ridge or fibrous ring encircling partially or completely the LVOT.2 Regar-ding the associated defects, SAS can be classified as simple (when it is isolated) or complex (when it is asso-ciated with other congenital defects of the structures corresponding to the left heart).3 The pathogenesis of fixed SAS is still a debate topic, regarding the coexis-ting characteristics not only of a congenital disease but also of an acquired disease. Multiple studies showed the fact that there is a variable risk of restenosis after surgical removal, probably due to the persistence of the pathogenic mechanism postoperatively.4,5,6 Recent studies have demonstrated the existence of significant differences between the simple and the complex form of SAS regarding not only the short-term but also the long-term postoperative mortality.5,6
The present retrospective SAS study aimed to iden-tify the clinical and echocardiographic characteristics of patients with fixed SAS evaluated in our center, fo-cusing on the comparative analysis of the subgroups of patients with simple or complex SAS regarding echo-cardiographic findings, therapeutic management, clini-cal outcomes, and mortality.
METHODS
All patients with fi xed SAS evaluated at least once in the „Prof. Dr. C. C. Iliescu” Emergency Institute of Cardiovascular Diseases between January 1st, 2003 and December 31st, 2016 were identi fied using the ho-spital electronic database. Inclusion criteria were as follows: at least one diagnostic code related to sub-aortic area, a fi rm diagnosis of fixed SAS mentioned in any document stored in the electronic database, at least one transthoracic/transesophageal echocardio-graphic examination stored, with adequate assessment of SAS morphology, associated heart defects, cardiac chambers and left ventricular function quantification, existence of intraoperative description of SAS and technical approach for surgically managed patients. Exclusion criteria were as follows: subaortic stenosis with a dominant/exclusive dynamic component, in-traoperative description of asymmetric septal hyper-trophy, the complete absence of clinical and echocar-diographic data requested in this study.
We recorded clinical and echocardiographic data; moreover, National Health Database Platform was used to establish the current vital status of the selec-ted patients.
For the analysis of collected data, different sub-groups were used: the type of SAS (simple/complex), the treatment (surgical/conservative), the patient sta-tus (alive/deceased). The subgroups of patients with simple SAS and complex SAS respectively were com-pared and there were noticed differences regarding age, gender, NYHA functional class and left ventricle ejection fraction. In order to eliminate the confoun-ding factor created by these differences, the propen-sity score matching technique was used, nearest nei-ghbour matching, resulting in two similar subgroups.
Continuous data are expressed as mean ± standard deviation (SD) and categorical variables as absolute numbers percentages. Differences in measurements between groups were assessed using Student’s t-test, one way ANOVA or Mann-Whitney test for continu-ous variables and chi-square test for categorical vari-ables. A 2-tailed p-value <0.05 was considered statistically significant. Regression analysis was used for the correlation between variables. Statistical analysis was performed using SPSS version 19.0 (SPSS, Inc., Chica-go, IL).
RESULTS
From the 132 patients with SAS initially identifi ed, only 93 patients met the study criteria and formed the study group: 61 patients with simple SAS (66%) and 32 patients with complex SAS (34%). The patients were followed-up for 42.4 (5.68-76.37) months.
Demographic, clinical and morphological data. The demographic characteristics of the study group are presented in Table 1.
The majority of patients (69.4%) were classified in NYHA functional class II at fi rst evaluation, both in simple (67.2%) and complex (74.1%) SAS subgroups. There were no asymptomatic patients in the complex group. The severity of symptoms (angina, dyspnoea, palpitations) at fi rst evaluation did not significantly correlate with age at that moment (r=-0.04, p=0.66) or with subaortic mean gradient (r=0.12, p=0.25).
Regarding the morphological type, in 96.8% of the cases, the echocardiographic/intraoperative aspect was suggestive for a fibro-muscular diaphragm or ridge; there was one case with tunnel-like obstruction and two cases with accessory mitral tissue. For the complex SAS, the presence of four congenital associa-ted heart defects was noted: ventricular septal defect (37.5%), aortic valve anomaly (congenital aortic ste-nosis, bicuspid aortic valve) (32.5%), aortic coarctati-on (17.5%) or mitral valve anomaly (congenital mitral stenosis, parachute mitral valve) (12.5%). In the majo-rity of cases, SAS was associated with a single other congenital heart defect (81.3%); there were two cases with three associated congenital defects: congenital mitral stenosis, a bicuspid aortic valve with aortic ste-nosis and coarctation of the aorta, in other words, a Shone complex.
Echocardiographic data
The main echocardiographic parameters measured are displayed in Table 2. There were no significant di-fferences between the simple SAS and complex SAS subgroups (Table 2). The mean distance between the aortic valve and the obstructive diaphragm/membrane was 7.8 ± 4.2 mm.
The severity of the subaortic obstruction was quan-tifi ed using Doppler peak and mean systolic pressure gradients (Table 2). The peak subaortic gradient in the studied group had a mean value of 69.06 (±34.94) mm Hg and a median value of 66.5 (40-94) mm Hg; the mean subaortic gradient had a mean value of 44.03 (±23.37) and a median value of 39.5 (25-60.25) mm. The peak gradient values in simple SAS group appear to be higher than in the complex group, without re-aching a statistical significance (72 vs. 65.5, p=0.66); the same situation is valid for the mean subvalvular gradient values (44.5 vs. 37, p=0.7).
The mean subaortic gradient significantly correla-ted with interventricular septum thickness (R2=0.14, p=0.001), both in the entire study group, as shown in Figure 1, as in each SAS subgroup, with a better correlation coeffi cient in the complex SAS subgroup (R2=0.18, p=0.03) than in the simple SAS subgroup (R2=0.11, p=0.01). Also, there was a significant cor-relation between mean subaortic gradient and left ventricle posterior wall thickness (R2=0.22, p<0.001) (Figure 2), with a correlation coeffi cient similar in the complex group (R2=0.24, p=0.02) and the simple group (R2=0.23, p<0.001).
Mean subaortic systolic gradient also correlated with relative wall thickness (R2=0.28, p<0.001), in
the entire SAS population and both SAS subgroups, as illustrated in Figure 3A and Figure 3B, respectively. There was no correlation with left ventricle mass, nei-ther in the entire group (p=0.44), nor in each subgroup separately.
Regarding the complications of subaortic stenosis, we noticed that 75.3% of the patients had a certain degree of secondary aortic regurgitation at first eva-luation; there were more patients with severe aortic regurgitation at first evaluation in the complex SAS group (30%) compared with the simple SAS group (11.9%). There was no significant correlation between
age or mean subaortic gradient at first evaluation and the degree of aortic valvular dysfunction.
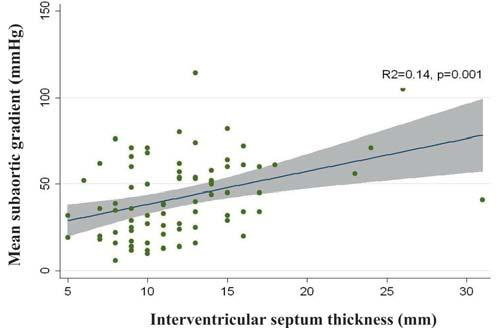
Figure 1. Correlation between mean subaortic gradient and interventric-ular septum thickness in the entire study group.
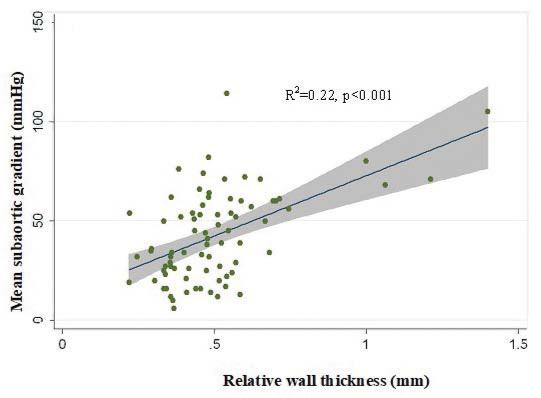
Figure 2. Correlation between mean subaortic gradient and posterior wall thickness in the entire study group.
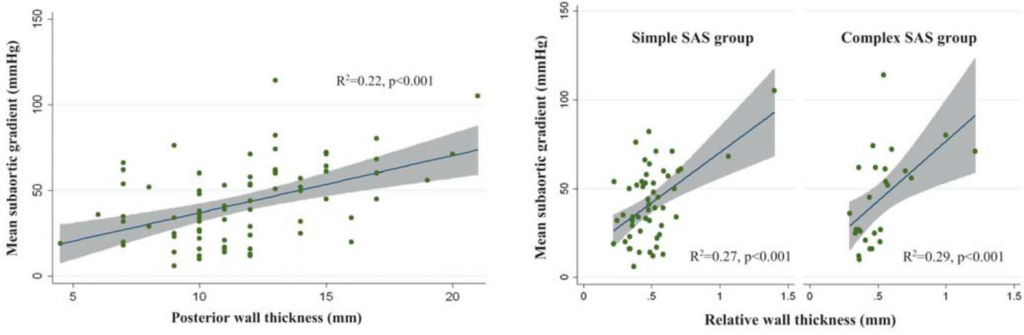
Figure 3. Correlation between mean subaortic gradient and relative wall thickness in the entire study group (A) and in both subgroups (B).
Treatment data.
As far as treatment is concerned, 32 patients (34.4%) had a surgical correction, where-as 61 patients (65.6%) were treated conservatory or refused surgery. There were 19 patients in the simple SAS group (31.1%) who required surgical intervention in comparison with 13 patients in the complex SAS group (40.6%) (p=0.36).
The mean values of peak and mean subaortic gradient at first evaluation for operated and conservatively treated patients are illustrated in Figure 4; both peak and mean subaortic gradients have significant greater values for the patients who underwent surgery.
Outcome data.
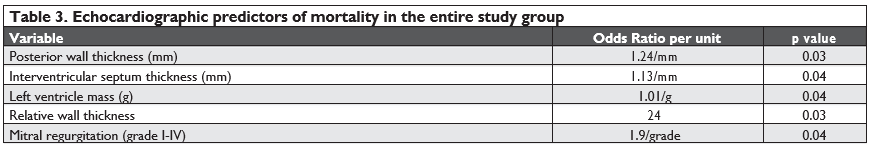
During the mean follow-up of 42.4 (5.68-76.37) months, there were 12 deaths in the entire group, with a mortality rate of 12.9%; the-re were no signifi cant differences between the two subgroups (13.11% in the simple SAS group vs. 12.5% in the complex SAS group, p=0.93). Echocardiographic predictors of mortality were: posterior wall thickness (p=0.03), interventricular septum thickness (p=0.04), left ventricle mass (p=0.04), relative wall thickness (p=0.03), mitral regurgitation (p=0.04), as seen in Ta-ble 3.
Propensity score matching analysis.
The findings of the comparative analysis of simple and com-plex SAS subgroups may be influenced by age, gender, NYHA functional class and left ventricular ejection fraction differences. For decreasing the impact of the-se differences, we used an adjustment method- pro-pensity score matching technique, nearest neighbour matching, resulting in two similar subgroups regarding age (mean 26 years), gender (52% females), NYHA functional class (second 75%, third 9%) and left ven-tricular ejection fraction (mean 61%) at diagnosis: 20 patients with simple SAS and 24 patients with complex SAS.
Left ventricular diastolic diameter (51 vs. 47, p=0.08) and interventricular septum thickness (12 vs. 10, p=0.08) had higher values in the complex SAS group, even though there is a similar mean aortic gra-dient in the two subgroups (40 vs 45 mmHg, p=0.4). There was no significant difference of left ventricular ejection fraction between the two subgroups (60% vs 61%, p=0.4). We analysed the values of left ventricular mass obtained for the two balanced subgroups; there was a difference not only between simple and complex SAS groups (287 ± 167 g vs. 402 ± 229 g, p=0.08), but also between operated and conservatively managed patients (389 ± 219 g vs. 297 ± 188 g, p=0.17), but without reaching a statistical significance. Moderate/severe mitral regurgitation seemed more frequent in the complex SAS group in comparison with simple SAS group (25% vs. 10%), without statisti-cal significance, while moderate/severe aortic regurgi-tation had an equal prevalence in both subgroups (54% vs 55%).
There were significantly more patients who re-ceived surgical treatment in the complex SAS group in comparison with simple SAS group (54% vs. 25%, p=0.05, Figure 5), with replacement of the aortic valve in 38% of the cases in the complex group, while there was no aortic valve replacement in the simple one.
Mortality rate seemed to be higher in the complex SAS group compared with simple SAS group (16% vs. 10%, p=0.4), both for operated patients (15% vs 0%, p=0.5) and conservatively treated patients (18% vs 13%, p=0.6), without reaching statistical significance, most probably because of the small number of pati-ents.
Mean subaortic gradient at first evaluation in com-plex SAS group significantly correlated with left ventricular end-diastolic diameter (negative correlation, R2=0.22, p=0.04), interventricular thickness (positi-ve correlation, R2=0.22, p=0.03) and posterior wall thickness (positive correlation, R2=0.25, p=0.02); the-re were no signifi cant correlations in the simple SAS group (Figure 6A, B, C). There is also a negative cor-relation between mean subaortic gradient and aortic ring dimension (R2=0.25, p=0.001), with a correlation coeffi cient lower in the complex SAS group (R2=0.16, p=0.06) than in the simple SAS group (R2=0.48, p=0.002).
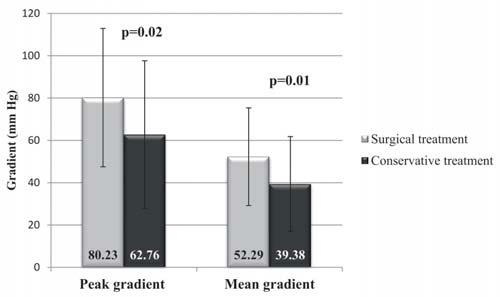
Figure 4. Peak and mean subaortic gradient in SAS patients according to the type of treatment.
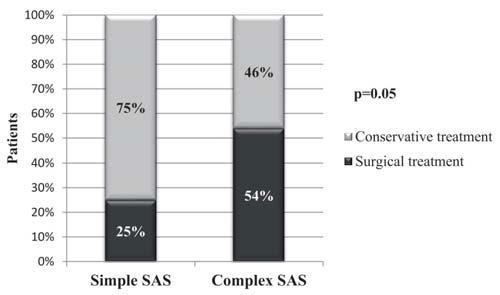
Figure 5. The distribution of patients who were surgically/conservatively managed in the two subgroups of SAS patients.
DISCUSSION
To the best of our knowledge, this is the first study performed in a general cardiology setting in Romania to focus on clinical and echocardiographic characteris-tics of fi xed subaortic stenosis, classifi ed as simple or complex, conservatively or surgically treated.
Regarding clinical aspects, only 4.7% of patients were asymptomatic at first evaluation, most of the patients being classifi ed in NYHA functional class II. The percentage of asymptomatic patients in other studies was reported to be 70% or 61.9% but also 15%.4-6 The-se differences might be caused by the value of mean subaortic gradient at first evaluation which was lower in the fi rst two studies than in the third one or they may be explained by the fact that symptomatic pati-ents were addressed more frequently to our Institute. The severity of symptoms at first evaluation did not significantly correlate with age at that moment or with subaortic mean gradient, an observation also made by Brauner et al.7
In most cases, the subaortic stenosis consisted of a fibro-muscular diaphragm or ridge, similar to other studies which established a predominance of this form in 84-91%.7,8 Regarding associated congenital heart de-fects, the most frequent one was the ventricular septal defect, in 46.9% of complex cases; similar results have been reported by other studies: 37-48%. 7,9 Congenital defects of the aortic valve were noted in 40.6% of ca-ses; the incidence of the bicuspid aortic valve was 25%, similar to other reports in the literature:16-32%.7,10,11 There were two cases of Shone syndrome; Van der Linde et al. reported in their study which involved 149 patients two cases of Shone syndrome.12
Considering the entire group, the peak subaortic gradient had a mean value of 69.0 ± 34.9 mmHg, hi-gher than the value obtained by Van der Linde et al. in their study.12
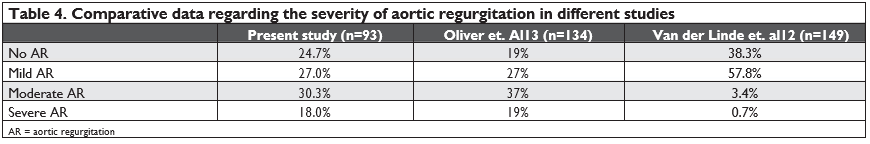
Regarding subaortic stenosis complications, 75.3% of patients had a certain degree of aortic regurgitation at first evaluation; other studies reported quite similar values of 61.7%, 75% or 81%.6,1213 Comparative ele-ments regarding aortic regurgitation in our study and in other two studies are presented in Table 4. There are authors supporting the idea that there is a signifi-cant relationship between the severity of obstruction (quantified by the subaortic systolic gradient) and the degree of aortic regurgitation in children and adults and that one can prevent secondary aortic valvular involvement by surgical intervention when the gradi-ent exceeds 30 mmHg.5,13,14,15 We found no significant correlation between mean subaortic gradient at first evaluation and the severity of aortic regurgitation; fu-ture studies are required to clarify this aspect, as the severity of aortic regurgitation still represents an indi-cation for surgery.16
Both peak and mean subaortic gradients had signifi – cant greater values at first evaluation in patients who underwent surgery, compared with the patients who were treated conservatively (80/53 mmHg vs. 52/34 mmHg, p=0.01); these results are close to the ones reported by another study which showed a signifi cant difference between mean value of peak subaortic gra-dient in operated patients (41.9 ± 19.9 mm Hg) and in conservatively treated patients (28.4 ± 14.1 mm Hg).12
Analysing the balanced subgroups using the propen-sity score matching method, we found that left ven-tricular diastolic diameter and interventricular septum thickness have higher values in the complex SAS group, even though there is a similar mean aortic gradient in the two subgroups; this result could be an indicator of the possible role played by associated congenital de-fects in the left ventricle remodelling. Moderate/seve-re aortic regurgitation had an equal prevalence in both subgroups, but aortic valve replacement was perfor-med only in patients with complex SAS; this fact might also suggest the existence of an additive effect of the associated cardiac defects on aortic valve dysfunction.
The rate of mortality seems to be higher in the complex SAS group compared with simple SAS group (16% vs. 10%, p=0.4), both for operated patients (15% vs. 0%, p=0.5) and conservatively treated patients (18% vs. 13%, p=0.6), but without reaching a statistical significance due to the small number of patients. The-se results are similar to those obtained by Dorobantu et al. who reported a higher mortality rate in patients with complex SAS both for early postoperative mor-tality and long-term mortality.17
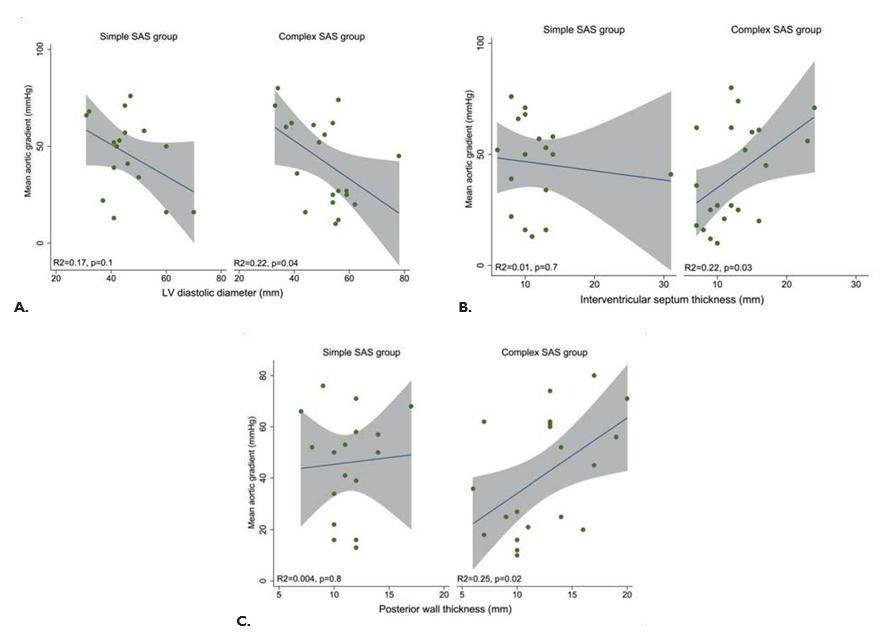
Figure 6. Correlation between mean subaortic gradient and left ventricular end-diastolic diameter (A), interventricular septum thickness (B) and poste-rior wall thickness (C) in both adjusted subgroups of SAS patients.
Study limitations
The main limitation of this study is its retrospective character; the available information can differ from a patient to another taking into consideration the 13 year-interval settled. The follow-up of the initially selected patients was insufficient, thus becoming an in-clusion/exclusion criterion. Another study limitation is the lack of left ventricle dimensions indexing; in this case propensity score matching technique was used.
CONCLUSION
Complex form of subaortic stenosis is associated with a more important left ventricular remodelling proba-bly due to the role played by associated cardiac de-fects and seems to have a less favourable outcome, both in surgically managed and conservatively treated patients, independent of NYHA functional class and left ventricular ejection fraction at initial diagnosis, with the limitation of the small dimension of the stu-died group. Further larger and prospective studies are needed in order to confirm these aspects.
Conflict of interest: none declared.
References
1. Ghiorghiu I, Enache R. Boli cardiace congenitale. În: Ginghină C. Mic tratat de cardiologie. Editura Academiei Române, Bucureşti, 2010; 555-590.
2. Macdonald ST, Walker F. Subvalvular and Supravalvular Aortic Ste-nosis. În: Gatzoulis MA, Webb GD, Daubeney PEF. Diagnosis and Management of Adult Congenital Heart Disease. Philadelphia: Else-vier/Saunders; 2011; 236-242.
3. McMahon Colin. Left Ventricular Outflow Obstructions: Aortic Valve Stenosis, Subaortic Stenosis, Supravalvular Aortic Stenosis and Bicuspid Aortic Valve. În: Moller JH, Hoffman JIE, Benson DW, Van Hare GF, Wren C. Pediatric Cardiovascular Medicine, 2nd Edition. John Wiley & Sons; 2012; 406-425.
4. Rayburn ST, Netherland DE, Heath BJ. Discrete membranous sub-aortic stenosis: improved results after resection and myectomy. Ann Thorac Surg 1997;64:105-109.
5. Darcin OT, Yagdi T, Atay Y, et al. Discrete subaortic stenosis: surgi-cal outcomes and follow-up results. Tex Heart Inst J 2003;30:286-292.
6. Sadeghian H, Karimi A, Ahmadi SH, et al. Discrete Subvalvular Aor-tic Stenosis: Severity of Aortic Regurgitation and Rate of Recurrence at Midterm Follow-Up after Surgery. J Tehran Univ Heart Cent 2008;3:219–224.
7. Brauner R, Laks H, Drinkwater DC, Shvarts O, Eghbali K, Galindo A. Benefi ts of early surgical repair in fi xed subaortic stenosis. J Am Coll Cardiol 1997;30:1835–1842.
8. Newfeld EA, Muster AJ, Paul MH, Idriss FS, Riker WL. Discrete sub-valvular aortic stenosis in childhood. Study of 51 patients. Am J Car-diol 1976;38:53-61.
9. Leichter DA, Sullivan I, Gersony WM. “Acquired” Discrete Subval-vular Aortic Stenosis: Natural History and Hemodynamics. J Am Coll Cardiol 1989;14:1539-1544.
10. Choi JY, Sullivan ID. Fixed subaortic stenosis: anatomical spectrum and nature of progression. Heart 1991;65:280-286.
11. Geva A, McMahon CJ, Gauvreau K, Mohammed L, del Nido PJ, Geva T. Risk Factors for Reoperation After Repair of Discrete Subaortic Stenosis in Children. J Am Coll Cardiol 2007;50:1498-1504.
12. van der Linde D, Takkenberg JJM, Rizopoulos D, et al. Natural his-tory of discrete subaortic stenosisin adults: a multicentre study. Eur Heart J. 2013;34(21):1548-1556.
13. Oliver JM, González A, Gallego P, Sánchez-Recalde A, Benito F, Mesa JM. Discrete subaortic stenosis in adults: increased prevalence and slow rate of progression of the obstruction and aortic regurgitation. J Am Coll Cardiol 2001;38:835–842.
14. Wright GB, Keane JF, Nadas AS, Bernhard WF, Castaneda AR. Fixed subaortic stenosis in the young: medical and surgical course in 83 pa-tients. Am J Cardiol 1983;52:830–835.
15. Coleman DM, Smallhorn JF, Williams WG, Freedom RM, McCrindle BW. Postoperative Follow-Up of Fibromuscuiar Subaortic Stenosis.
J Am Coll Cardiol 1994;24:1558-1564.
16. Stout KK, Daniels CJ, Aboulhosn JA, et al. 2018 AHA/ACC Guide-line for the Management of Adults With Congenital Heart Disease. Journal of the American College of Cardiology Aug 2018.
17. Dorobantu DM, Sharabiani MT, Martin RP, et al. Surgery for simple and complex subaortic stenosis in children and young adults: Results from a prospective, procedure-based national database. J Thorac Cardiovasc Surg 2014;148:2618-2626.
 This work is licensed under a
This work is licensed under a