T. Constantinescu, M. Bogdan
Article received on the 18th of May 2013. Article accepted on the 27th of May 2013.
“Marius Nasta” Institute of Pneumology, Bucharest, « Carol Davila » University of Medicine and Pharmacy, Bucharest
Tudor Constantinescu, MD “Marius Nasta” Institute of Pneumology, Bucharest, «Carol Davila» University of Medicine and Pharmacy, Bucharest
Abstract: Chronic thromboembolic pulmonary hypertension (CTEPH) represents a distinct category of pulmonary hypertension (PH), forming the 4th group of the Dana Point PH Classification 2008. CTEPH is considered a rare complication of pulmonary embolism (PE) but the incidence and prevalence are still unclear. Although the natural history of the disease is severe, more than 50% of the CTEPH patients may be cured by thrombendarterectomy. For the inoperable or persistent postoperatory CTEPH patients medical treatment with modern vasodilators used in pulmonary arterial hypertension (PAH) may represent a solution.
CTEPH is characterized by a mechanical obstruction of the pulmonary arteries by organized thrombi. The disease is associated with a variable degree of vascular hypertensive remodelling of the pulmonary microcirculation, leading to an increase in pulmonary vascular resistance, pulmonary pressures and finally to progression towards right heart failure.
The confirmation requires the evaluation in an experienced centre and should include an invasive hemodynamic profile, as well as complex imaging investigations, such as scintigraphy, angioCT or MRI, for establishing the surgical indication, and, if required, preoperative conventional angiography.
Presently, in Romania specific therapies are available for operable patients and also for inoperable patients, through the PAH National Program, with positive long term results.
Keywords: chronic thromboembolic pulmonary hypertension, thrombendarterectomy, specific therapies
Abstract: Hipertensiunea pulmonară cronică postembolică (HTPE) reprezintă o categorie distinctivă de hipertensiune pulmonară (HTP), formând grupul 4 al clasificării Dana Point 2008. HTPE este considerată a fi o complicație rară a tromboembolismului pulmonar (TEP) însă incidența şi prevalența reale sunt încă neclare. Deşi evoluția naturală a bolii este severă, peste 50% din pacienți pot fi vindecați prin trombendarterectomie. Pentru pacienții inoperabili sau cu HTP persistentă postoperatorie pot fi încercate terapiile vasodilatatoare utilizate în hipertensiunea arterială pulmonară (HTAP).
HTPE se caracterizează prin obstrucția mecanică a arterelor pulmonare prin trombi organizați. Boala se asociază într-o pondere variabilă cu remodelarea hipertensivă a microcirculației pulmonare determinând creşterea rezistenței vasculare pulmonare, a presiunii în arterele pulmonare şi progresia către insuficiență cardiacă dreaptă.
Diagnosticul de certitudine necesită evaluarea într-un centru cu experiență şi presupune atât bilanțul hemodinamic invaziv cât şi evaluarea operabilității prin investigații imagistice complexe: scintigrafie, angioCT sau RMN iar preoperator, angiografie convențională.
In România există posibilități terapeutice atât pentru pacienții operabili cât şi pentru cei ce necesită terapie vasodilatatoare pulmonară prin Program National, cu bune rezultate pe termen lung.
Cuvinte cheie: Hipertensiune pulmonară cronică postembolică, trombendarterectomie, vasodilatatoare pulmonare.
Definition and history
The first observations, regarding a severe evolution of patients with PH that associated obstructions in the pulmonary arteries of large calibre, were made during the ‘50. In 1970, in the San Diego centre, K. Moser and N. Braunwald performed the first successful pulmonary thrombendarterectomy6.
In 1973 the first WHO World Symposia on PH took place in Geneva, event during which the basis for the first PH classification was laid, and thus knowledge about this rare disease started growing7. During the following years the diagnostic criteria for CTEPH were refined, aiming to make a difference from the other forms of PH (idiopathic or associated) and also to develop clear thrombendarterectomy indications.
The diagnostic criteria, established by the International Registry on patients with CTEPH, according to the present international guidelines on PH, are the following: evidence of pulmonary vascular obstructions at ventilation/perfusion scintigraphy, contrast computed tomography (angioCT) or conventional pulmonary angiography in a patient with a minimum of 3 months of anticoagulant therapy and precapillary PH at right heart catheterism (RHC) (mPAP >25 mmHg and pulmonary wedge pressure PCP <15 mmHg). The presented criteria are completed by the confirmation of a total pulmonary vascular resistance (TPVR) of >4 Wood Units and the presence of functional limitation NYHA class II-IV1,2.
The natural history, in the absence of specific therapy, has a severe prognosis despite chronic anticoagulation. A mortality of 80% at 2 years was observed in CTEPH patients with mean pulmonary arterial pressure (mPAP) >50 mmHg and another trial, showed a 90% mortality at 3 years in patients with mPAP >30 mmHg29,30.
Physiopathology
Most of the studies accept the hypothesis of a trigger event represented by pulmonary thromboembolism (PE). The rare evolution towards CTEPH is a consequence of incomplete thrombus resolution, without any evidence in such patients of a fibrinolysis deficit or of an over-activation of the thrombosis cascade3-5. Nevertheless, in a significant number of patients the initial thrombembolic event cannot be identified, the proportion of this category of patients varying among studies: 23% in the International Registry (679 patients enrolled)2, respectively 63% in another series of 143 patients8. In cases without a documented trigger event, the hypothesis of an „in situ” thrombosis should be taken into consideration. The time length between the trigger thrombembolic event (clinically manifest or silent) and the development of CTEPH is also variable, from several months to several years11.
The main physiopathologic features of CTEPH are: organized, fibrotic, white thrombi, located intraluminal or adherent to the vascular wall, at the level of the proximal pulmonary artery tree, and vascular remodelling at the level of the pulmonary microcirculation, represented by obstructive thrombotic lesions at arteriolar level and by arteriopathic lesions. The latter are characterized by proliferation and obstruction in the distal arterioles through intimal hyperplasia, medial hypertrophy and adventicial fibrosis, including plexiform lesions similar to those found in idiopathic PAH. Microvascular disease develops also in unobstructed teritories, due to shear stress imposed by blood redistribution in the lungs, as well as distally from central vascular obstructions, due endothelial dysfunction in these underperfused teritories9.
The presence of microvascular disease is primarily responsible for disease progression and represents the main surgical contraindication when high vascular resistances are found, leading to failure of thrombendarterectomy in 10-15% of cases (persistent or recurrent CTEPH). On the other hand, microvascular disease might be targeted by vasodilator therapies used in the treatment of PAH.
The main risk factors associated to CTEPH are: the presence of ventriculo-atrial shunts, implantable pacemakers, Klippel Trenaunay syndrome and chronic inflammatory diseases, such as osteomyelitis and intestinal inflammatory disease (Crohn disease and ulcerative colitis)12,13. Patients with recurrent, high risk, or idiopathic PE and those with PE at young age have a higher risk for developing the disease after a first episode of PE2,14.
Regarding coagulation and fibrinolysis, patients with CTEPH had more frequently high titres of antiphospholipidic antibodies and factor VIII, compared to PAH patients and respectively to the general population. No significant modifications of thrombin, C and S protein or factor V Leyden levels were noticed in those patients15,16.
Epidemiology
In the past CTEPH was considered an extremely rare disease. In 1985 only 85 cases worldwide were reported. In 1990 Moser identifies 250 cases and estimates an incidence of the disease of 0.1%, reported to the survivors of a first episode of PE17,18. Due to the rise in the diagnostic rate of PE, through the development of various diagnostic means, the contemporary incidence for PE is 0.01-0.05% in the general population20. In 2001 Jamieson appreciates an incidence of 0.1-0.5% reported to the PE survivors, corresponding to an estimate case number in the USA of 500-250019. Later on, 2 studies followed prospectively through echocardiography, populations with clinically manifest PE and reported a higher incidence, of 5.1% (Ribeiro 78 patients) and 3.8% (Pengo – 223 patients)21,22. In these studies CTEPH was defined as PH (sPAP >40 mmHg and mPAP >25 mmHg, confirmed through RHC), in patients with a first episode of PE and recently unexplained dyspnoea at exertion. The previously presented incidence estimations didn’t include patients who developed CTEPH in the absence of clinically evident PE2,8, therefore suggesting a prevalence of the disease clearly higher than the historical one. Nevertheless, CTEPH remains an often under diagnosed clinical entity, as was confirmed by a British populational study, which summarized data collected between 2001 and 2006, from 5 PH expert centres and one national centre dedicated to thrombendarterctomy. Although incidence of CTEPH, as presumed by prospective studies indicated 1000 possible new cases of CTEPH in the UK/ year22, diagnosis was confirmed in only 100 new patients/year, with an incidence of 1,75/million inhabitants/year and a prevalence of 5,83/million inhabitants23.
Diagnosis
Clinical practice has noticed 2 types of presentation1: insidious beginning symptoms, with progressive dyspnoea at exertion, palpitations, thoracic pain, syncope and sometimes signs of right heart failure in a patients with PE history and2 presence of symptoms outside a clinically identifiable episode of PE, the diagnosis being established after a workup for PH.
The recommended paraclinical investigations can be divided into the following categories25-28:
A. standard investigations for PH suspicion, such as: ECG, pulmonary radiography, pulmonary function testing and echocardiography, the latter confirming the suspicion of PH by estimating the pressure gradient between the right ventricle (RV) and the right atrium (RA).
B. the standard investigation for the confirmation of precapillary PH: RHC
C. imaging investigations, for the aetiology confirmation of CTEPH: classically, the ventilation/perfusion scan (V/P) and the pulmonary angiography represented the diagnostic standard, but recently, sectional methods such as CT and MRI tend to become of first intention.
V/P scan – can identify the presence of isolated or multiple perfusion defects, segmentary or subsegmentary, with high sensibility and specificity for CTEPH. Also it can differentiate between proximal and distal obstruction. In the case of small, diffuse, subsegmentary defects, the differential diagnosis with other diseases, such as idiopathic PAH may be difficult. A normal pattern in the V/P scan has high negative predictive value (practically 100%) for CTEPH1. In Romania, due to the lack of ventilation scan, perfusion scan can be interpreted only in association with a normal thoracic radiography.
Contrast CT – the apparition in 1992 of spiral CT, and after 2000 of multi detector CT, have imposed CT as a reference diagnostic tool (Papworth algorithm for the diagnosis of CTEPH)24, with a negative predictive value of 99% and also with high sensibility and specificity, but in addition offering the possibility of an accurate differential diagnosis with diseases of the pulmonary parenchyma25. The CT scan can identify (Figure 1):
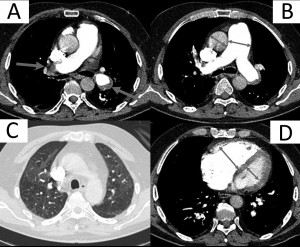
Figure 1. Computed tomography of the thorax with iv contrast in a CTEPH patient: A – main pulmonary arteries’ obstruction by intramural organized thrombotic material; B – dilatation of the pulmonary trunk; C – lung window with mosaic pattern due to blood redistribution in un-obstruated teritories; D – right heart dilatation with compression of the interventricular septum.
A. modifications at the level of the pulmonary arteries, down to subsegmentary level:
a. calibre modifications– parietal thickening of pulmonary arteries and post-stenotic dilations
b. the absence of intimal linearity at the level of the pulmonary arteries
c. the presence of intraluminal thrombi
d. the presence of webs of thrombotic material in the vascular lumen
e. the complete absence of an arterial branch due to proximal occlusion
B. other specific modifications:
a. the mosaic pattern of pulmonary parenchyma, secondary to the hypoperfusion of obstructed zones and the compensatory hyperperfusion of unobstructed ones.
b. dilation of collateral bronchial circulation
c. the presence of sequelar subpleural post PE lesions
C. non-specific modifications of PH:
a. right heart dilation and rectilinear position of the interventricular septum
b. the dilation of the pulmonary artery (diameter of pulmonary trunk compared to aorta > 0,8)
Magnetic resonance imaging (contrast angio-MRI) identifies, similarly to CT, vascular obstructions down to segmentary level, but can offer supplementary information regarding right ventricular function, cardiac output and should be preferred when monitoring without cumulative irradiation is needed.
Conventional angiography represents an invasive method, difficult, expensive and with high morbidity risks with relatively low availability. Nevertheless, it offers extensive information about the morphology of the pulmonary arterial tree (road map). Usually, angiography is reserved only for preoperative evaluation for thrombendarterctomy.
Right heart catheterism allows the measurement of pulmonary pressures (especially mPAP and capillary wedge pressures PCP), of vascular resistances (especially TPVR) and of cardiac output (CO). TPVR correlates linearly to per operative mortality after thrombendarterctomy (4% when TPVR <900 dyne s cm-5 vs. 20% when TPVR >1200 dyne s cm-5)35,36 and CO is one of the most important prognostic parameters in PH1.
The evaluation of CTEPH severity is similar to that of PAH, reuniting clinical data (NYHA functional class), data from the 6MWT (exercise capacity), echocardiographical parameters (conventional parameters such as RA dilation, TAPSE, the presence of pericardial effusion, and Tissue Doppler Imaging parameters) and RHC parameters (RA pressure, TPVR and cardiac index)1,31-33.
Treatment
The unanimous opinion indicates nowadays thrombendarterctomy as first line therapy in CTEPH. 20-50% of patients cannot benefit from this intervention, either due to inaccessibility of the lesions, or due to comorbidities1,2,34. For this category of patients medical therapies can be used.
General measures and conventional treatment are the same with those used in PAH and include: prevention of influenza and pneumococcal infections through vaccination, diuretic therapy and oxygen therapy in selected patients. Unlike in PAH, in CTEPH oral anticoagulation is mandatory lifelong, including in operated patients, and requires a target INR of 2-31 or, according to some authors of 2,5-3,534. Pulmonary transplantation represents a reserve solution, similarly to PAH, although usually CTEPH patients are older, therefore limiting the indication. The treatment algorithm proposed for CTEPH is described in Scheme 1.
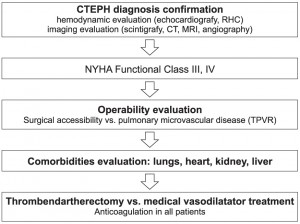
Scheme 1. Treatment algorithm in CTEPH.
Surgical treatment
Thrombendarterectomy has been under continuous development during the last 40 years. Due to the complexity of the procedure and the reduced number of patients, current guidelines recommend the referral of patients towards expert centres. Contemporary there are approximately 20 such centres worldwide, in most of the cases being just one centre per country (eg. France, UK, Austria).
The protocol of the intervention was elaborated in the San Diego University and includes median sternotomy, switching to extracorporeal circulation and afterwards a sequential incision of the main pulmonary arteries at intrapericardic level. Due to the multiple systemic-pulmonary shunts through angiogenesis (transdiaphragmatic, intercostal and bronchial circulation), the special preparation of the patient implies hypothermia of 18-20°C and cardiac arrest (limited intervals of 10-20 minutes) throughout the desobstruction of the each pulmonary arteries. The desobstruction requires a dissection plane (endarterectomy) at the level of the pulmonary artery media, plane which is continued downstream to the subsegmentary level with the help of video-assisted procedures, thus eliminating the thrombotic organized material incorporated in the structure of the vessel wall10,18,34.
Indications of operability were established through consensus by the European and Canadian International Registry, which included almost 700 patients with CTEPH2:
– symptomatic functional limitation, NYHA III,IV
– significant PH, mPAP >25 mmHg, TPVR >4uW
– surgically accessible pulmonary obstruction
– the absence of major operative contraindications, especially severe COPD or left ventricular dysfunction
– the absence of significant pulmonary microvascular disease, RPT <18uW
The per operative mortality has lowered significantly during the last decades, from 15-24% at the beginning of the procedures to 5-10% contemporary. Centres with high experience, such as Paris (Hopital Marie Lannelongue – presently the centre with the highest number of patients operated/ year worldwide) or San Diego report figures for per operative mortality of 1-3%. According to the International Registry the mean per operative mortality among the 384 operated patients was 4,7%2.
In a majority of operated patients a significant and durable lowering of pulmonary resistances occurs, those patients being considered cured and having only an indication of permanent anticoagulation. 10-15% of patients present postoperatory persistent or recurrent PH, probably due to the microvascular disease, and do not have an indication for reintervention10,18,34. These patients are candidates for medical therapy.
Medical treatment
The introduction of medical therapies in CTEPH was based on physiopathological and functional similarities between the microvascular disease in CTEPH and PAH, especially idiopathic PAH, condition in which medical therapy showed efficiency in controlled clinical trials.
The therapeutic indication is meant for some distinct categories of patients1,2,10,34:
– Patients with surgical inaccessible lesions (distal)
– Patients with important comorbidities and high surgical risk
– Patients with persistent/recurrent CTEPH after thrombendarterctomy
– Patients with a surgical accessible obstruction but high values of the TPVR and thus high surgical risk, patients in which medical therapy represents a «bridge» towards surgical intervention.
Pulmonary vasodilator therapies used in PAH rely on 3 physiopathological pathways: (A) the prostacyclin pathway for epoprostenol and its derivates, treprostinil, iloprost and beraprost, (B) the endothelin pathway for the endothelin receptor antagonists, such as bosentan, ambrisentan and soon macitentan and (C) the nitric oxide (NO) pathway for the phosphodiesterase 5 inhibitors, such as sildenafil, tadalafil and in the near future the soluble guanylate cyclase activators such as riociguat. Presently, clinical studies also evaluate tyrosine kinase inhibitors, such as imatinib1,38.
The 2009 ESC/ERS PH Guidelines, reconfirmed at the 5th World Symposia on PH, Nice 2013, assume a level of indication of IIbC for pulmonary vasodilator therapies in CTEPH due the lack of evidence in controlled clinical trials1.
Epoprostenol was evaluated in 2 retrospective series of patients. Bresser39 evidenced a slight hemodynamic benefit on 9 patients on bridge therapy before thrombendarterectomy and Cabrol40 noticed an increase in the 6MWT, as well as an amelioration of hemodynamic parameters, persistent at one year, in 27 patients with inoperable CTEPH. Treprostinil was evaluated in monocentric uncontrolled study in 28 patients with severe inoperable CTEPH with significant clinical and functional benefit41. The AIR study, placebo controlled, focused on iloprost efficiency but didn’t demonstrate a significant benefit neither on the composite primary endpoint, that included NYHA functional class and 6MWT, nor on hemodynamic parameters42.
Reichenberger evaluated the evolution of 104 patients on sildenafil for inoperable CTEPH and noticed a stable benefit at one year on the 6MWT, as well as on cardiac output and TPVR43.
Bosentan was the first medication to be studied in a placebo controlled trial in CTEPH. The BENEFIT study included 157 patients with inoperable or residual / recurrent CTEPH after thrombendarterectomy, but the primary endpoints weren’t accomplished. TPVR dropped significantly (with 22%), but no benefit was observed regarding the distance at the 6MWT44.
Riociguat, a soluble guanilat cyclase stimulator has entered recently clinical trials and induced a significant amelioration of the 6MWT in a subgroup of 42 patients, included in an uncontrolled phase II study (45). The positive results of the placebo controlled trial CHEST, which included 261 patients with inoperable CTEPH; are currently in print.
Combination vasodilator therapy and lung transplantation may also be considered in CTEPH, similarly to PAH, although there are no evidences to support this approach. According to the therapeutic algorithm, after the CTEPH confirmation, to evaluate the operability before initiating pulmonary vasodilator therapies (Scheme 1). Medical treatment may also be implied in selected cases, after surgery, in case of persistent / recurrent CTEPH.
The International Registry on CTEPH
Between 2007 and 2009 all consecutive patients with confirmed diagnosis of CTEPH, from 26 centres in 16 countries (Europe and Canada), were included in the prospective International Registry, and were followed for at least 10 months. The Registry has enrolled a total number of 679 patients, thus becoming the largest CTEPH registry. It should be mentioned that Romania was present in this Registry, through the “Marius Nasta” Institute of Pneumology from Bucharest.
Among the important data emerging from the Registry, it should be underlined that 63% of patients have been considered operable and 57% have undergone thrombendarterectomy. PE was present in the medical history of 74,8% of patients, a much higher value than previous observations, being more frequent and recurrent in operable patients. A good correlation between conventional angiography (indicating operability in 63% of cases) and angioCT (indicating operability in 60% of cases) was noticed. The per operative mortality was 4,7%.
At diagnosis, 37,9% of patients were already on at least one major vasodilator therapy (variability between countries 2,2-88,9%) with 28,3% in the operable group and 53,8% in the inoperable group. We should emphasise that preoperative vasodilator therapy has a reduced effect on hemodynamic, and no effect at all on postoperative evolution, but can delay the referral of the patient towards a surgical expert centre46.
National situation in Romania (from the perspective of a PH centre)
During the last years several solutions have become available in our country for patients with CTEPH. Thrombendarterecomy is now feasible at the Timisoara Institute of Cardiovascular Diseases (surgical team lead by Prof. Dr. Marian Gaspar in collaboration with Prof. dr. Walter Klepetko from AKH Viena, the centre with the highest experience in this field in Central Europe)
Medical treatment with specific pulmonary vasodilators has become possible through the National Program for Pulmonary Arterial Hypertension. Its inclusion criteria are PAH and inoperable CTEPH or persistent postoperative CTEPH. The National Program includes 5 adult expert centres and 4 paediatric expert centres. In 2012 the program counted 254 patients on treatment with Sildenafil, Bosentan or a combination of both. In 2010 27% of patients treated through the National Program had a diagnosis of CTEPH, 37% had idiopathic PAH and 31% had PAH associated to congenital heart disease37.
The “Marius Nasta” Institute of Pneumology provides follow up for 19 patients with CTEPH, of whom 10 were operated through thrombendarterectomy and 11 receive medical vasodilator therapy, among these being 2 patients with persistent postoperative CTEPH.
Conclusions
CTEPH represents a pathology with a continuously
growing prevalence, especially due to the development of diagnostic methods and the rise in awareness throughout the medical world. Nevertheless, it remains frequently under diagnosed and has a major impact on morbidity and mortality.
The confirmation requires the evaluation in an experienced centre and should include an invasive hemodynamic profile, as well as complex imaging investigations, such as scintigraphy, angioCT or MRI, for establishing the surgical indication, and, if required, preoperative conventional angiography.
Presently, in Romania specific therapies are available for operable patients and also for inoperable patients, through the PAH National Program, with positive long term results.
Conflict of interests: The authors declare that they was consultants, speakers and investigators in clinical trials for Actelion, Pfizer and GlaxoSmithKline companies. No conflict of interest exists regarding this article.
Refferences
1. Galie N. et al. Guidelines for the diagnosis and treatment of pulmonary hypertension. European Heart Journal. 2009; 30: 2493–2537.
2. Pepke-Zaba I, Delcroix M, Lang I et al. Chronic Thromboembolic Pulmonary Hypertension: Results From an Internaţional Prospective Registry. Circulațion 2011; 124:1973-1981.
3. Peacock A, Simonneau G, Rubin L. Controversies, uncertainties and future research on the treatment of CTEPH. Proc Am Thorac Soc 2006; 3:608–614.
4. Egermayer P, Peacock AJ. Is pulmonary embolism a common cause of CTEPH? Limitations of the embolic hypothesis. Eur Respir J 2000; 15:440–448.
5. Moser KM, Bloor CM. Pulmonary vascular lesions occurring in patients with chronic major vessel thromboembolic pulmonary hypertension. Chest 1993; 103: 685–692.
6. Newman JH Pulmonary Hypertension, AJRCCM 2005; 172:1072-1077.
7. Hatano S, Strasser R. editors. Primary pulmonary hypertension. World Health Organization, Geneva, 1975
8. Lang I. CTEPH — Not So Rare after All. N Engl J Med 2004; 350:2236-2238.
9. Galie N, Kim N. Pulmonary Microvascular Disease in CTEPH. Proc Am Thorac Soc 2006; 3:571–576.
10. Mayer E, Klepetko W. Techniques and Outcomes of Pulmonary Endarterectomy for CTEPH Proc Am Thorac Soc 2006; Vol 3: 589–593.
11. Manecke GR Jr, Wilson WC, Auger WR, et al. CTEPH and pulmonary thromboendarterectomy. Semin Cardiothorac Vasc Anesth 2005; 9:
189-204.
12. Bonderman D, Wilkens H, Wakounig S, et al. Risk factors for CTEPH. Eur Respir J 2009; 33: 325–331.
13. Bonderman D, Jakowitsch J, Adlbrecht C et al. Medical conditions increasing the risk of CTEPH. Thromb Haemost 2005; 93:512–516.
14. Pengo V, Lensing AW, Prins MH et al. Incidence of CTEPH after pulmonary embolism. N Engl J Med 2004; 350:2257–2264.
15. Wolf M, Boyer-Neumann C, Parent F et al. Thrombotic risk factors in pulmonary hypertension. Eur Respir J 2000; 15:395–399.
16. Bonderman D, Turecek PL, Jakowitsch J et al. High prevalence of elevated clotting factor VIII in CTEPH. Thromb Haemost 2003; 90:372–376.
17. Dalen JE, Alpert JS. Natural history of pulmonary embolism. Prog Cardiovasc Dis. 1975; 17:259.
18. Moser KM, Auger WR and Fedullo PF. Chronic major-vessel thromboembolic pulmonary hypertension. Circulațion. 1990; 81:1735-1743
19. Fedullo PF, Auger WR, Kerr KR. Chronic Thromboembolic Pulmonary Hypertension. N Engl J Med 2001; 345:1465-1472
20. Naess IA, Christiansen SC, Romundstad P, Incidence and mortality of venous thrombosis: A population-based study. J Thromb Haemost. 2007; 5:692-699.
21. Ribeiro A, Lindmarker P, Johnsson H. Pulmonary Embolism: One-Year Follow-Up With Echocardiography Doppler and Five-Year Survival Analysis. Circulation. 1999;99:1325-1330
22. Pengo V, Lensing A, Prins M. Incidence of CTEPH after Pulmonary Embolism. NEJM 2004; 350:2257–2264
23. Condliffe R, Kiely DJ, Gibbs S. Improved Outcomes in Medically and Surgically Treated CTEPH. AJRCCM 2008; 177:1122-1127.
24. Coulden R. State-of-the-Art Imaging Techniques in CTEPH Proc Am Thorac Soc 2006; 3:577–583.
25. Compendiu de boli cardiovasculare, Editia a IIIa, Vol II, sub redactia Prof. Dr. Maria Dorobantu, Cordul pulmonar cronic: 1025-1036
26. Ginghina C, Popescu BA, Jurcut R. Esentialul in Ecocardiografie. Editura Medicala Antaeus 2005
27. Ginghina C. Hipertensiunea pulmonara in practica de cardiologie. Editura Academieie Romane Bucuresti, 2006
28. Ginghina C. Mic tratat de cardiologie. Editura Academiei Romane, 2010
29. Dartevelle P, Fadel E, Mussot S. Chronic thromboembolic pulmonary hypertension. Eur Respir J 2004; 23:637–648
30. Azarian R; Wartski M; Collignon MA. Lung perfusion scans and hemodynamics in acute and chronic pulmonary embolism. The Journal of nuclear medicine, 1997; 38:980-983
31. Jurcut R, Giusca S, La Gerche A. The echocardiographic assessment of the right ventricle: what to do in 2010? Eur J Echocardiogr 2010; 11:81-96
32. Giusca S, Jurcut R, Ghiorghiu I, Ginghina C. Comparison of right ventricular function between different etiologies of pulmonary hypertension. Romanian Journal of Cardiology 2010, vol XXV, Supp.A, A166-167
33. S. Giusca, Ruxandra Jurcut, Carmen Ginghina Echocardiographic assessment of hemodynamics comparison with cardiac catheterization. Romanian Journal of Cardiology 2010, vol XXV, Supp.A, A235-236
34. Mayer E. Surgical and post-operative treatment of CTEPH. Eur Respir Rev 2010; 19:64–67.
35. Riedel M, Stanek V, Widimsky J, Prerovsky I. Long-term follow-up of patients with pulmonary thromboembolism: Late prognosis and evolution of hemodynamic and respiratory data. Chest 1982; 81:151-158.
36. Lewczuk J, Piszko P, Jagas J et al. Prognostic factors in medically treated patients with chronic pulmonary embolism. Chest 2001; 119:818-823.
37. Constantinescu T, Zaharia DC, Bogdan MA. The National Program of Pulmonary Hypertension in Romania, past, present and future. ERS Congress 2010, P1132
38. Humbert M, Sitbon O, Simonneau G. Treatment of PAH. N Engl J Med 2004; 351:1425-1436
39. Bresser P, Fedullo PF, Auger WR. Continuous intravenous epoprostenol for CTEPH. Eur Respir J 2004; 23:595–600
40. Cabrol S, Souza R, Jais X et al. Intravenous epoprostenol in inoperable CTEPH. J. Heart Lung Transplant. 2007 ; 26:357–362.
41. Skoro-Sajer N, et al. Treprostinil for severe inoperable CTEPH. J Thrombosis Hemostatis 2007; 5:483-489.
42. Olschewski H, Simonneau G, Galiè N. Inhaled Iloprost for Severe Pulmonary Hypertension for the Aerosolized Iloprost Randomized Study Group. N Engl J Med 2002; 347:322-329
43. Reichenberger F, et al. Long-term treatment with sildenafil in CTEPH. Eur Resp J 2007; 30:922-927.
44. Jaïs X, D’Armini AM, Jansa P. Bosentan for treatment of inoperable chronic thromboembolic pulmonary hypertension: BENEFiT (Bosentan Effects in iNopErable Forms of chronIc Thromboembolic pulmonary hypertension), a randomized, placebo-controlled trial. J Am Coll Cardiol. 2008;52:2127-2134
45. Ghofrani HA, Hoeper MM, Halank M. Riociguat for CTEPH and PAH: a phase II study. Eur Respir J 2010; 36: 792-799
46. Jensen KW, Kerr KM, Fedullo PF. Pulmonary hypertensive medical therapy in CTEPH before pulmonary thromboendarterectomy. Circulation 2009; 120:1248-1254.
 This work is licensed under a
This work is licensed under a