Sebastian Onciul1, Sven Plein2
1 Cardiovascular Magnetic Resonance Imaging Fellow, Leeds University, United Kingdom
2 PhD, MD Germany, MRCP, Professor of Cardiology, British Heart Foundation Senior Clinical Research Fellow & Honorary Consultant Cardiologist, Head of the Division of Biomedical Imaging, University of Leeds, United Kingdom
Abstract: Coronary artery disease (CAD) is the leading cause of cardiovascular mortality worldwide. Non-invasive di-agnosis of CAD is essential for lowering the number of unnecessary invasive coronary angiograms (ICA). Due to the low diagnostic accuracy of electrocardiographic exercise testing (EET), the current practice has shifted to the use of non-inva-sive imaging testing where available. Cardiovascular magnetic resonance (CMR) is unique among the current non-invasive imaging tests because it offers equally accurate information about cardiac morphology, function, myocardial ischaemia and viability in one single examination, without exposing patients to ionizing radiation. This is particularly important in complex clinical scenarios such as patients with multi-vessel CAD, total chronic occlusions or prior revascularization, in which preci-se knowledge of ischaemic territories, viability and left ventricular (LV) function are necessary to determine the appropriate treatment. This review is focused on myocardial perfusion CMR imaging while briefly discussing the other CMR methods relevant to the assessment of CAD. We also provide practical information on how to perform stress myocardial perfusion CMR as well as the current guideline indications of CMR in CAD detection.
Keywords: CMR, cardiovascular magnetic resonance, fi rst pass perfusion, coronary artery disease, non-invasive imaging
THE CURRENT ROLE OF NON-INVASIVE IMAGING FOR CAD DETECTION
Invasive coronary angiography (ICA) remains the definitive test for anatomic definition of coronary stenosis and with the addition of fractional fl ow reserve (FFR) to determine the functional signifi cance of coronary lesions. However ICA is not a risk-free procedure and recent data show that a large number of ICAs performed in routine practice are unnecessary. A study including almost 400 000 patients showed that almost 60% of patients without known CAD who underwent elective ICA were found to have no obstructive CAD1. Performing non-invasive testing can avoid unne-cessary ICA in patients with suspected CAD. The-refore, the current European guidelines recommend non-invasive testing for patients at intermediate risk of CAD. Anatomical techniques are excellent for ruling out CAD but do not provide information regarding the functional significance of detected coronary obstruc-tions. For example, CTA is able to precisely localize coronary stenosis but does not provide information regarding their functional signifi cance, unless combi-ned with expensive computational modeling methods such as computed tomography-derived fractional flow reserve (CT-FFR)2,3. However, revascularization is generally recommended only if ischaemia or LV dys-function are demonstrated, so that functional testing is required to guide the management of patients with CAD.
Historically, electrocardiogram exercise testing (EET) has been used as a first line functional test to screen patients presenting with chest pain for the pre-sence of significant CAD. However, as the sensitivity of ETT has been reported to be as low as 45-50% when referral bias is considered4,5,6 it can no longer be considered as an appropriate fi rst line test for CAD detection. The European guidelines recommend that EET should not be employed in patients with a pre-test probability >65%4. In clinical practice, EET is in-creasingly replaced by stress imaging tests including stress echocardiography, CMR, myocardial perfusion scintigraphy (MPS), computed tomography angiogra-phy (CTA) or positron emission tomography (PET).
THE CURRENT APPLICATIONS OF CMR IN CAD
Among the non-invasive imaging modalities, CMR stan-ds out because it provides both structural and quanti-tative functional information without patient exposure to ionizing radiation and without limitations relating to the patient’s body habitus. Historic contraindications such as implantable cardiac devices are being overco-me through the development and increasing use of MR safe and conditional devices7. Ischaemia detection is only one of the many appli-cations of CMR in CAD. Using cine imaging in repro-ducible orientations and with whole heart coverage, CMR is currently considered the reference method for quantification of left ventricle (LV) and right ven-tricle (RV) dimensions and function. Furthermore, CMR has the unique capacity of tissue characterization. Among the available CMR methods, T2 weighted CMR is sensitive to myocardial oedema and late gadolinium enhanced (LGE) CMR detects myocardial necrosis or scar with very high contrast and spatial detail. Using these methods, in the setting of acute myocardial infarction, CMR can delineate ir-reversible damage from the area at risk providing an accurate estimation of the final infarct size; it can also detect microvascular obstruction and intramyocardial hemorrhage, all of which have proved important pro-gnostic value in this population8. In the context of acute myocardial infarction with non-obstructed coronary arteries, CMR can provide the fi nal diagnosis in up to 70% of cases, differentiating between myocarditis, myocardial infarction, Tako-Tsubo or other cardiomyopathies, with a significant clinical impact on patient management9. In chronic CAD, CMR can establish the location and extent of myocardial scar and can identify lypomatous metaplasia, a finding associated with high arrhythmic risk. The transmurality of myocardial scar is a measure of myocardial viability and predicts functional recovery after revascularization10. Finally, ischaemia by CMR can be detected with ei-ther vasodilator stress myocardial perfusion CMR or inotropic stress wall motion imaging. In short, CMR is an anatomical and functional test able to offer a multitude of complex information wi-thin a single examination.
DIAGNOSTIC ACCURACY OF STRESS PERFUSION CMR FOR THE DETECTION OF CAD
Stress perfusion CMR has demonstrated high accuracy for the detection of CAD in several studies. When using invasive FFR as the reference standard, stress CMR demonstrated a sensitivity and specificity of 82% and 94% respectively, with an area under the recei-ver-operator characteristic curve of 0.92 to detect coronary stenosis at a threshold of FFR <0.75 in one of several recent single-centre studies11. Similarly, a meta-analysis of 14 studies showed that with FFR as the reference standard, the diagnostic ability of stress CMR to detect CAD is high with pooled sensitivity and specificity of 90% and 87% respectively12.
The largest prospective, real world randomized study that assessed the diagnostic accuracy of CMR in detecting CAD was the CE-MARC study, which re-cruited 752 patients who underwent CMR and MPS with ICA as the reference standard for CAD detecti-on. The study clearly showed superiority of CMR over MPS for detection of CAD. For CMR the sensitivity was 86.5%, specificity 83.4%, positive predictive value 77.2%, and negative predictive value 90.5%13. The sen-sitivity of MPS was 66.5%, specifi city 82.6%, positive predictive value 71.4%, and negative predictive value 79.1%13. CE-MARC was a single centre study, but si-milar results were obtained in the multi-centre, mul-tivendor MR-IMPACT 2 study. In this study, the sen-sitivity of CMR to detect CAD was superior to MPS, while its specificity was inferior to MPS14.
PROGNOSTIC INFORMATION FROM STRESS PERFUSION CMR
In several single-centre studies, stress perfusion CMR has demonstrated a high negative predictive value for major adverse cardiac events (MACE). A meta-analysis of 14 of these studies, recruiting 12 178 patients iden-tified a negative predictive value for MACE of a nor-mal CMR of 98.12% during a mean follow-up of 25.3 months15. This translates into an event rate after a negative stress CMR scan of 1.03% per annum whi-ch is similar to that of a normal population. On the other hand, a positive stress CMR was associated with a 3-fold increased risk of MACE15.
Five-year follow-up of the CE-MARC study indica-tes that compared with MPS, stress perfuiosn CMR is a stronger predictor of risk for MACE, independent of cardiovascular risk factors, angiography result, or initial patient treatment16.
TECHNICAL ASPECTS OF STRESS CMR
The most widely used CMR method for CAD detec-tion is fi rst-pass perfusion imaging during vasodilator stress, and this review refers only to this technique. Dobutamine stress CMR is another stress CMR method, but it is less performed worldwide.
FIRST-PASS PERFUSION CMR – HOW DOES IT WORK?
As the name implies, the first–pass perfusion tech-nique is based on visualization of myocardial perfusion defects immediately after the intravenously injec-ted Gadolinium based contrast agents (GBCA) rea-ches the LV cavity and enters the myocardium. The contrast can be visualized as entering the right heart chambers and then entering the left heart chambers, and finally entering the myocardium which becomes brighter in proportion to the amount of contrast upta-ke in the myocardium (Figure 1). Areas with reduced myocardial perfusion will receive less GBCA and will therefore appear darker than normally perfused regi-ons which will appear brighter as they receive relati-vely more GBCA.
Usually, basal, mid and apical short axis LV slices are acquired in each cardiac cycle, allowing assessment of 16 of the 17 segments in the American Heart Association (AHA) classification. The addition of a long axis view is feasible, although rarely used in clinical practice, to also cover the apical segment. Conventionally, data are acquired over 40-60 consecutive cardiac cycles to accurately assess the persistence of any perfusion de-fect after contrast administration.
In order to detect ischaemia, the image acquisition is performed during maximal hyperemia induced by infusion of a vasodilator agent. A rest study is usu-ally performed 15 minutes before or after the stress imaging, using the same CMR sequence, slice positions and contrast dose, but in the absence of vasodilatation (Figure 3).
STRESS AGENTS IN CLINICAL USE
Adenosine, regadenoson and dypiridamole are the vasodilators used in first-pass perfusion CMR. Ade-nosine acts on 4 receptors A1, A2A, A2B and A3, while regadenoson is a selective A2A receptor ago-nist. Dypiridamole acts by inhibiting adenosine reup-take and thus increasing the availability of endogenous adenosine17. Adenosine and regadenoson have similar coronary vasodilator potency and both are superior to dypiridamole17.
Due to its ultra-short half-life (<10 seconds), ade-nosine effects are neutralized immediately after its infusion is stopped making the administration of the antidote almost redundant17.
Early studies from the nineties showed a low in-cidence of adverse reaction with adenosine infusion even when 6 minutes protocols were employed18. More recently, very large registry data showed even lower incidence of adverse events when the stress agent was administered according to currently recom-mended protocols. European cardiovascular magnetic resonance (EuroCMR) registry gathered data from more than 27000 patients from 57 centers in 15 coun-tries. Mild complications occurred in 3.6%, and severe complications in only 0.026% of patients19. Although traditionally adenosine was contraindicated in pati-ents with asthma or chronic obstructive pulmonary disease, evidence from clinical trials have shown that adenosine is safe in patients with history of mild, well-controlled lung obstructive disease20.
In terms of symptoms patients may experience chest pain, shortness of breath, facial flushing, lighthea-dedness, diaphoresis, or a metallic taste during adeno-sine infusion. These symptoms are transitory, usually lasting less than one minute.
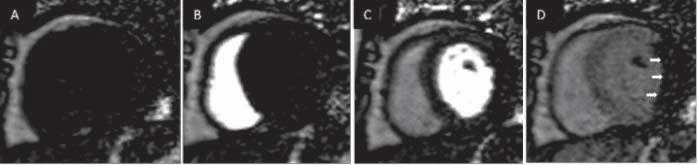
Figure 1. Example of a first pass perfusion CMR study (only the mid slice is shown). The acquisition begins before contrast is present in the heart cham-bers (A). Contrast can be first visualized entering the right ventricle (B) then the left ventricle (C) and finally it enhances the myocardium (D). In this case a myocardial perfusion defect is observed in the lateral and infer-lateral walls (white arrows).
GADOLINIUM BASED CONTRAST AGENTS
Perfusion CMR uses T1 weighted pulse sequences, meaning that the contrast between different tissues is based on their different T1 relaxation times. Due to its paramagnetic properties, Gadolinium shortens T1 relaxation time thus it enhances contrast betwe-en tissues with different relaxation properties. In the context of first-pass perfusion imaging, the normally perfused myocardial segments will appear brighter than the abnormal segments, due to T1 prolonging effects of GBCA.
There are 2 structurally distinct categories of GBCA: linear or macrocyclic. The latter are consi-dered safer because the Gadolinium ion is more ti-ghtly attached to a chelating molecule and thus it is less likely to dissociate. Lower dissociation rates are translated into lower risk of gadolinium deposition in various organs of the body. GBCA are not nephro-toxic, however exposure to linear GBCA has been associated with nephrogenic systemic fibrosis (NSF), an extremely rare but serious disease of fibrosis of the skin and internal organs. No cases of NSF have been reported in patients with normal renal function and macrocyclic contrast agents appear to be much less likely to cause NSF. Because of concerns over tissue retention, the European Medicines Agency has recently recommended the suspension of the marketing autho-rizations for linear GBCA.
ABOUT THE PHYSICS
First-pass perfusion imaging can be performed on both 1.5 and 3.0 Tesla MRI scanners. A higher magnetic fi-eld strength is associated with increased signal to no-ise and contrast to noise ratio which can be used to boost spatial and/or temporal resolution. However, 3.0 Tesla imaging is also more challenging due to its inherent susceptibility to artifacts due to greater mag-netic field inhomogeneity.
The pulse sequence used for first-pass perfusion is made up of a saturation recovery preparation pulse followed by a fast imaging technique for read out such as steady state free precession (SSFP), spoiled gradient echo (GRE) or echo planar imaging (EPI). The satura-tion recovery preparation pulse has the role of enhan-cing contrast between the low GBCA uptake areas and the normally perfused myocardium. Methods such as non-cartesian sampling, parallel imaging, par-tial-Fourier and spatio-temporal undersampling with methods such as k-t Broad Linear Speed up Technique (k-t BLAST) have been introduced to accelerate data acquisition and allow acquisition of higher spatial re-solution data.
In a small pilot study, high-resolution perfusion-CMR using k-t BLAST (1.6×1.6 mm in-plane) had higher overall diagnostic accuracy than standard-resolution acquisition (2.5×2.5 mm in-plane) for the detection of CAD in both single- and multivessel disease21. Further-more a reduction in endocardial dark-rim artifact was reported with high-resolution perfusion images. These methods require further validation in larger cohorts.
A TYPICAL CMR PROTOCOL FOR SUSPECTED OR KNOWN CAD
An example of a CMR protocol indicated in patients with suspected or known CAD is depicted in Figure 2. This protocol aims to identify stress induced myocar-dial ischaemia and myocardial viability during a 45-50 minutes scan as well as providing an assessment of LV contractile function and general cardiac morphology.
Usually the study begins with a low resolution sur-vey scan of the chest and upper abdomen which allows for localization of the heart within the thorax. Then, orthogonal slices through the heart are obtained simi-lar to those in conventional echocardiography: two-chamber, four-chamber and short-axis views. During this stage, the basal, mid and apical short axis slices are planned for use in subsequent first-pass perfusion imaging.
The next step consists in intravenous infusion of the vasodilator agent. The infusion rates are 140 μg/kg/ min for Adenosine and 0.56 mg/kg for dypiridamole. Regadenosone is injected as a single 400 μg bolus (fi-xed dose, no need weight adjustment). When adequa-te stress is obtained, a bolus of contrast (0.05-0.1 mmol/kg body weight) is injected at a rate of 3-7 ml/s, followed by a saline chasing bolus (30 ml). Immediately the three short axis slices previously prescribed are acquired in every cardiac cycle.
After approximately 10-15 minutes delay to allow wash out of contrast agent from the myocardium, rest perfusion images are acquired in the same slices, using the same sequence and the same dosage of GBCA, the only difference being the absence of vasodilator stress. Between stress and rest acquisitions, a stack of short axis cine images covering the whole left ventricle is acquired for functional analysis. Six-to-eight mm thick slices are acquired with a 2-4 mm slice gap, the num-ber of slices depending on the long axis dimension of the LV. This acquisition offers an exhaustive coverage of the whole LV providing accurate measurements of LV dimensions and function.
After the rest perfusion imaging, an optional bolus of GBCA may be administered (top-up) immediately after which the early Gadolinium enhancement (EGE) images are acquired; these allow for identification of intracavitary thrombus or evidence of MVO in the context of an acute ischaemic event.
Seven-to-ten minutes after the last dose of GBC-CA, late gadolinium enhancement (LGE) images are acquired to detect myocardial scar or fibrosis.
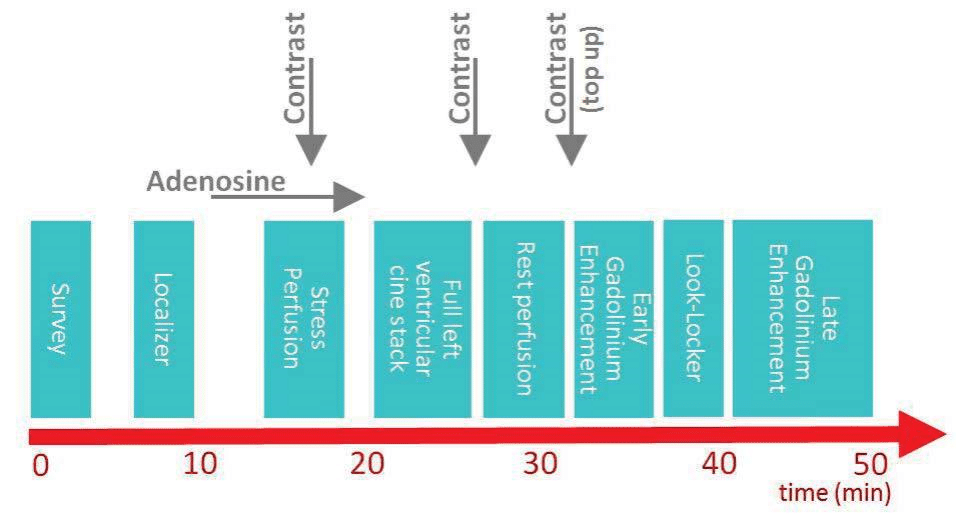
Figure 2. An example of a first pass perfusion CMR protocol. In this particular case, the total dose of contrast is administered divided in 3 equal doses: first during stress perfusion, second during rest perfusion and the last just before the early gadolinium enhancement imaging. This protocol provides information regarding left ventricular dimension and function, myocardial ischaemia and viability.
ARTIFACTS AND PITFALLS IN REPORTING FIRST-PASS PERFUSION IMAGING
As with all functional imaging techniques, accurate detection of ischaemia requires adequate stress. In-sufficient vasodilator stress (ie. caffeine antagonism) can result in false negative results. In clinical practice it is accepted that adequate stress is obtained when the heart rate increases with 10% and/or the blood pressures decreases with 10 mmHg compared to the pre-vasodilator administration values. Symptom occurrence is a confirmation that the stress agent is effective. Furthermore, adequate stress can be objec-tively assessed by the presence of splenic switch-off, which is the attenuation of splenic perfusion in the stress images compared with the corresponding rest images, due to splenic vasoconstriction by adenosi-ne22. As the spleen is usually included in the short axis views acquired during first-pass perfusion imaging, the splenic-switch off is a straightforward method to ob-jectify adequate vasodilator stress. Regadenoson does not produce a splenic switch-off phenomenon, as it acts selectively on A2 receptors in coronary micro-circulation with no effects on renal, mesenteric, or peripheral circulation.
The dark rim artifact is defined as a transient low signal line localized at the interface between blood pool and myocardium during the early phases of a myocardial perfusion study. The phenomenon is tem-porary, as it disappears after myocardial enhancement has occurred (after 8-10 cardiac cycles). The accurate differentiation between a true perfusion defect and dark rim artifact requires experience. A true myocar-dial perfusion defect persists longer, and is usually cha-racterized by a larger width, generally accompanied by a gradient from darker subendocardial towards a brighter subepicardial area with variable transmural extent. True perfusion defects also correspond to a coronary artery distribution.
Aliasing artifacts due to respiratory motion (parallel imaging artifacts) are associated with parallel imaging techniques, which are usually employed for shorter acquisition time. This technology works by under-sampling in the phase encoding direction and con-sequently the region outside the field of view wraps around in the middle of the image. If this artifact is not correctly recognized it might be misinterpreted as a myocardial perfusion defect (Figure 4). The artifact can be avoided by reducing the acceleration factor, or by increasing the field of view in order to encompass the entire chest.
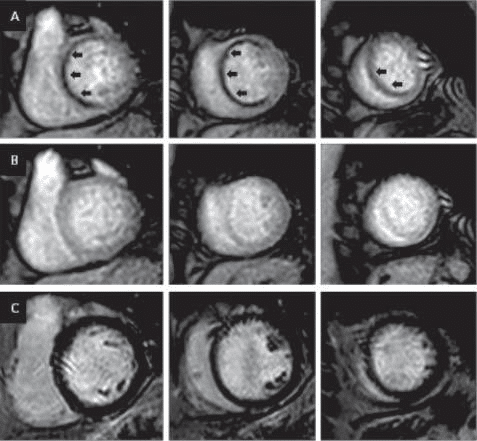
Figure 3. Example of a CMR study in suspected CAD. Basal, mid and apical short axis perfusion slices are acquired during stress (A). An extensive myo-cardial perfusion defect (black arrows) is noted in the basal anterior, antero-septal and infero-septal segments, mid anterior, antero-septal and infero-septal segments as well as in apical septal segment (in total 7 segments from 17). These perfusion defects are not visualized on rest acquisition (B). Late gadolinium enhancement imaging (C) does not show any evidence of scar/focal fibrosis, indicating that the myocardium is entirely viable with good outcome if revas-cularized.
EQUIPMENT AND STAFF REQUIREMENTS
An MRI scanner of minimum 1.5 Tesla is necessary for fi rst-pass perfusion imaging. The scanner should be equipped with a phased-array surface coil, as well as artifact resistant electrocardiogram ECG hardwa-re/software (e.g.vectorcardiogram) for efficient gating. An MRI-compatible power injector must be employed for contrast infusion. Resuscitation facilities (inclu-ding defibrillation/oxygen/suction) should be available as well as drugs to deal with potential reactions to GBCA and stressors.
Stress CMR can be performed and reported by either radiologists or cardiologists with appropriate expertise, depending on the national laws and regula-tions. In some countries cardiologists and radiologists report jointly, maximizing the benefits to patients. The European Society of Cardiology (ESC) and the European Society for Cardiovascular Radiology offer processes for certification in CMR. ESC Level 3 competency is re-quired for individuals wishing to perform, interpret, and report CMR studies fully independently, to lead a CMR laboratory and to supervise CMR training pro-grammes in an accredited CMR training centre. To obtain level 3 certifi cation an individual must spend at least 12 months under the supervision of a level 3 certified expert.
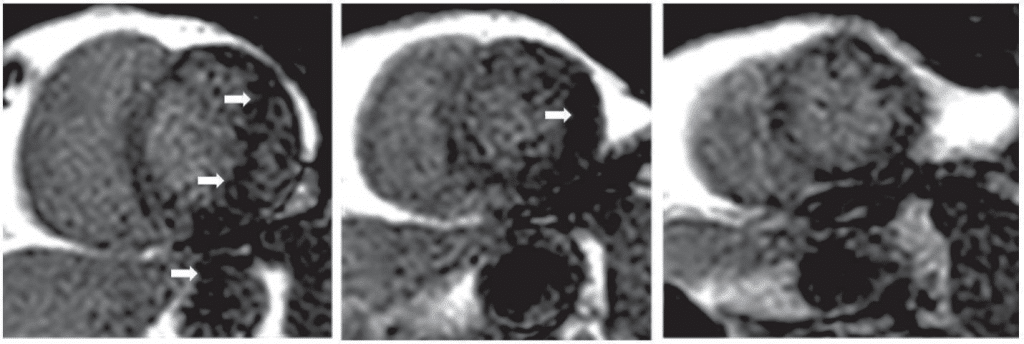
Figure 4. Example of a respiratory aliasing artifact (white arrows) on basal, mid and apical slices of a first pass perfusion CMR study. The dark appearance may be mistakenly interpreted as a perfusion defect.
EMERGING TECHNOLOGIES FOR ASSESSING MYOCARDIAL PERFUSION – THE QUANTITATIVE ASSESSMENT
In current clinical practice, myocardial perfusion de-fects are assessed qualitatively by distinguishing darker low-perfused areas from brighter normally perfused myocardium (see above). This approach is subjective and is highly dependent on the presence of differen-ces in signal intensities between healthy and ischaemic myocardium – in addition to substantial observer ex-perience. However in the presence of signifi cant ischaemia in all three coronary territories, the accurate visualization of perfusion defects may be challenging as there is no healthy reference myocardium to use as a visual comparator. Quantitative perfusion assessment by CMR can provide absolute myocardial blood flow (MBF) in units of ml/g/min. Myocardial perfusion reser-ve (MPR) can be calculated as the ratio between MBF during vasodilator stress and MBF at rest. Although historically quantitative perfusion analysis was associa-ted with long post-processing times, recently develo-ped technologies permit rapid, inline quantification of myocardial perfusion without prolonging scanning or reporting times23.
When compared to PET which is the gold standard imaging modality for non-invasive myocardial blood flow quantification, studies showed good correlation between MPR (CMR) and MPR (PET)23.
IS CMR THE BEST IMAGING MODALITY FOR DETECTING CORONARY ARTERY DISEASE?
As shown, first-pass perfusion CMR has several advan-tages over other non-invasive imaging modalities in di-agnosing CAD. First of all, it does not expose patients to ionizing radiation and offers good quality images in-dependently of the patient’s body habitus. Myocardial perfusion CMR imaging has better spatial resolution than MPS, thus allowing for detection of smaller per-fusion defects and has higher diagnostic accuracy than SPECT. CMR allows quantitative estimates of MBF comparable to PET.
Most importantly, CMR is unique among the other cardiac imaging techniques because of its multipara-metric nature. A single study provides information re-garding ventricular function, myocardial ischaemia, viability, and coronary artery anatomy. This is especially important in complex cases with multi-vessel disease, previous myocardial infarction and LV dysfunction in which the most suitable therapeutic strategy depends on demonstration of residual ischaemia, viability and correct estimation of LV ejection fraction. Subclinical anomalies of the intra-myocardial mecha-nics including torsion, twist, strain and strain rates can also be assessed by CMR11. The following are some clinical scenarios in which first pass perfusion might be indicated: Patients with suspected CAD with a pre-test probability of coronary artery disease of 15-85% according to ESC guidelines4.
Patients with abnormal resting ECG in which the ST changes during exercise are not interpretable or diffi cult to analyze: left bundle branch block, paced rhythm, pre-excitation, LV hypertrophy, electrolyte imbalance, and use of digitalis. Elec-trocardiographic exercise testing receives a class III indication in the European guidelines for pati-ents with ≥0,1mV ST-depression on resting ECG or taking digitalis4.
Patients with complex coronary pathology, multi-vessel disease, chronic total coronary occlusions, prior PCI/CABG, in which information regarding viability is necessary in order to indicate surgical/ interventional revascularization.
Patients with limited exercise capacity due to orthopaedic or other non-cardiac problems.
Patients with sub-optimal acoustic windows on echocardiography.
Women with suspected CAD. Exercise ECG testing demonstrated lower sensitivity and spe-cificity in women5. The CE-MARC study show-ed that CMR has greater sensitivity than MPS in both men and women. These findings, plus an absence of ionizing radiationexposure, mean that CMR should be more widely adopted in women with suspected CAD24.
Patients with known CAD in whom the interrup-tion of anti-ischaemic medication is not safe. As opposed to dobutamine stress imaging, CMR perfusion imaging does not require interruption of beta-blockers.
Patients in whom the high risk of malignant vern-tricular arrhythmias contraindicates dobutamine stress imaging.
CONCLUSION
By performing a CMR scan which usually lasts 45 minutes information regarding cardiac morphology and function, myocardial ischaemia and viability can be obtained. Taking into account its high diagnostic accu-racy and safety profile, stress perfusion CMR should be employed more often in the non-invasive detection of CAD. Currently the wide use of stress CMR is re-stricted by the low availability of scanners and adequa-tely trained doctors.
Funding: Sebastian Onciul was awarded with a European Society of Cardiology Training Grant in Cardio-vascular Magnetic Resonance Imaging.
References
1. M. R. Patel, E. D. Peterson, D. Dai, J. M. Brennan, R. F. Redberg, H.V. Anderson, R. G. Brindis, and P. S. Douglas, “Low diagnostic yield of elective coronary angiography.,” N. Engl. J. Med., vol. 362, no. 10, pp. 886–895, Mar. 2010.
2. C. A. Taylor, T. A. Fonte, and J. K. Min, “Computational fluid dyna-mics applied to cardiac computed tomography for noninvasive quan-tification of fractional flow reserve: scientific basis.,” J. Am. Coll. Cardiol., vol. 61, no. 22, pp. 2233–2241, Jun. 2013.
3. B. L. Norgaard, J. Leipsic, S. Gaur, S. Seneviratne, B. S. Ko, H. Ito, J. M. Jensen, L. Mauri, B. De Bruyne, H. Bezerra, K. Osawa, M. Marwan, C. Naber, A. Erglis, S.-J. Park, E. H. Christiansen, A. Kaltoft, J. F. Lassen,
H. E. Botker, and S. Achenbach, “Diagnostic performance of non-invasive fractional fl ow reserve derived from coronary computed tomography angiography in suspected coronary artery disease: the NXT trial (Analysis of Coronary Blood Flow Using CT Angiography: Next Steps).,” J. Am. Coll. Cardiol., vol. 63, no. 12, pp. 1145–1155, Apr. 2014.
4. G. Montalescot, U. Sechtem, S. Achenbach, F. Andreotti, C. Arden, A. Budaj, R. Bugiardini, F. Crea, T. Cuisset, C. Di Mario, J. R. Ferreira, B. J. Gersh, A. K. Gitt, J.-S. Hulot, N. Marx, L. H. Opie, M. Pfisterer,
E. Prescott, F. Ruschitzka, M. Sabate, R. Senior, D. P. Taggart, E. E. van der Wall, C. J. M. Vrints, J. L. Zamorano, S. Achenbach, H. Baumgartner, J. J. Bax, H. Bueno, V. Dean, C. Deaton, C. Erol, R. Fagard, R. Ferrari, D. Hasdai, A. W. Hoes, P. Kirchhof, J. Knuuti, P. Kolh, P. Lancellotti, A. Linhart, P. Nihoyannopoulos, M. F. Piepoli, P. Ponikowski, P. A. Sirnes, J. L. Tamargo, M. Tendera, A. Torbicki, W. Wijns, S. Windecker, J. Knuuti, M. Valgimigli, H. Bueno, M. J. Claeys, N. Donner-Banzhoff, C. Erol, H. Frank, C. Funck-Brentano, O. Gaemperli, J. R. Gonzalez-Juanatey, M. Hamilos, D. Hasdai, S. Husted, S. K. James, K. Kervinen, P. Kolh, S. D. Kristensen, P. Lan-cellotti, A. Pietro Maggioni, M. F. Piepoli, A. R. Pries, F. Romeo, L. Ryden, M. L. Simoons, P. A. Sirnes, P. G. Steg, A. Timmis, W. Wijns, S. Windecker, A. Yildirir, and J. L. Zamorano, “2013 ESC guideli-nes on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology.,” Eur. Heart J., vol. 34, no. 38, pp. 2949–3003, Oct. 2013.
5. A. P. Morise and G. A. Diamond, “Comparison of the sensitivity and specificity of exercise electrocardiography in biased and unbiased populations of men and women.,” Am. Heart J., vol. 130, no. 4, pp. 741–747, Oct. 1995.
6. V. F. Froelicher, K. G. Lehmann, R. Thomas, S. Goldman, D. Morri-son, R. Edson, P. Lavori, J. Myers, C. Dennis, R. Shabetai, D. Do, and J. Froning, “The electrocardiographic exercise test in a population with reduced workup bias: diagnostic performance, computerized interpretation, and multivariable prediction. Veterans Affairs Coo-perative Study in Health Services #016 (QUEXTA) Study Group. Quantitative Exercise Testing and Angiography.,” Ann. Intern. Med., vol. 128, no. 12 Pt 1, pp. 965–974, Jun. 1998.
7. R. J. Russo, H. S. Costa, P. D. Silva, J. L. Anderson, A. Arshad, R. W. W. Biederman, N. G. Boyle, J. V Frabizzio, U. Birgersdotter-Green, S. L. Higgins, R. Lampert, C. E. Machado, E. T. Martin, A. L. Rivard, J. C. Rubenstein, R. H. M. Schaerf, J. D. Schwartz, D. J. Shah, G. F. To-massoni, G. T. Tominaga, A. E. Tonkin, S. Uretsky, and S. D. Wolff, “Assessing the Risks Associated with MRI in Patients with a Pacema-ker or Defi brillator,” N. Engl. J. Med., vol. 376, no. 8, pp. 755–764, Feb. 2017.
8. E. Dall’Armellina, S. K. Piechnik, V. M. Ferreira, Q. L. Si, M. D. Ro-bson, J. M. Francis, F. Cuculi, R. K. Kharbanda, A. P. Banning, R. P. Choudhury, T. D. Karamitsos, and S. Neubauer, “Cardiovascular magnetic resonance by non contrast T1-mapping allows assessment of severity of injury in acute myocardial infarction,” J Cardiovasc Magn Reson, vol. 14, 2012.
9. A. G. Dastidar, J. C. L. Rodrigues, T. W. Johnson, E. De Garate, P. Singhal, A. Baritussio, A. Scatteia, J. Strange, A. K. Nightingale, G. D. Angelini, A. Baumbach, V. Delgado, and C. Bucciarelli-Ducci, “Myo-cardial Infarction With Nonobstructed Coronary Arteries,” JACC Cardiovasc. Imaging, Jan. 2017.
10. R. J. Kim, E. Wu, A. Rafael, E. L. Chen, M. A. Parker, O. Simonetti, F. J. Klocke, R. O. Bonow, and R. M. Judd, “The use of contrast-enhan-ced magnetic resonance imaging to identify reversible myocardial dysfunction,” N Engl J Med, vol. 343, 2000.
11. T. Lockie, M. Ishida, D. Perera, A. Chiribiri, K. De Silva, S. Kozerke, M. Marber, E. Nagel, R. Rezavi, S. Redwood, and S. Plein, “High-reso-lution magnetic resonance myocardial perfusion imaging at 3.0-Tesla to detect hemodynamically signifi cant coronary stenoses as determi-ned by fractional fl ow reserve.,” J. Am. Coll. Cardiol., vol. 57, no. 1, pp. 70–75, Jan. 2011.
12. M. Li, T. Zhou, L. Yang, Z. Peng, J. Ding, and G. Sun, “Diagnostic accuracy of myocardial magnetic resonance perfusion to diagnose ischemic stenosis with fractional flow reserve as reference: systema-tic review and meta-analysis.,” JACC. Cardiovasc. Imaging, vol. 7, no. 11, pp. 1098–1105, Nov. 2014.
13. J. P. Greenwood, N. Maredia, J. F. Younger, J. M. Brown, J. Nixon, C. C. Everett, P. Bijsterveld, J. P. Ridgway, A. Radjenovic, C. J. Dickin-son, S. G. Ball, and S. Plein, “Cardiovascular magnetic resonance and single-photon emission computed tomography for diagnosis of co-ronary heart disease (CE-MARC): a prospective trial.,” Lancet (Lon-don, England), vol. 379, no. 9814, pp. 453–460, Feb. 2012.
14. J. Schwitter, C. M. Wacker, N. Wilke, N. Al-Saadi, E. Sauer, K. Hu-ettle, S. O. Schonberg, A. Luchner, O. Strohm, H. Ahlstrom, T. Dill, N. Hoebel, and T. Simor, “MR-IMPACT II: Magnetic Resonance Imaging for Myocardial Perfusion Assessment in Coronary artery disease Trial: perfusion-cardiac magnetic resonance vs. single-pho-ton emission computed tomography for the detection of coronary artery disease: a comparative multicentre, multivendor trial.,” Eur. Heart J., vol. 34, no. 10, pp. 775–781, Mar. 2013.
15. P. Gargiulo, S. Dellegrottaglie, D. Bruzzese, G. Savarese, O. Scala, D. Ruggiero, C. D’Amore, S. Paolillo, P. Agostoni, E. Bossone, A. Soricelli, A. Cuocolo, B. Trimarco, and P. Perrone Filardi, “The pro-gnostic value of normal stress cardiac magnetic resonance in patients with known or suspected coronary artery disease: a meta-analysis.,” Circ. Cardiovasc. Imaging, vol. 6, no. 4, pp. 574–582, Jul. 2013.
16. J. P. Greenwood, B. A. Herzog, J. M. Brown, C. C. Everett, J. Nixon, P. Bijsterveld, N. Maredia, M. Motwani, C. J. Dickinson, S. G. Ball, and S. Plein, “Prognostic Value of Cardiovascular Magnetic Resonance and Single-Photon Emission Computed Tomography in Suspected Coronary Heart Disease: Long-Term Follow-up of a Prospective, Diagnostic Accuracy Cohort Study.,” Ann. Intern. Med., May 2016.
17. J. Layland, D. Carrick, M. Lee, K. Oldroyd, and C. Berry, “Adenosi-ne,” JACC Cardiovasc. Interv., vol. 7, no. 6, pp. 581–591, 2014.
18. M. D. Cerqueira, M. S. Verani, M. Schwaiger, J. Heo, and A. S. Iskan-drian, “Safety profile of adenosine stress perfusion imaging: Results from the adenoscan multicenter trial registry,” J. Am. Coll. Cardiol., vol. 23, no. 2, pp. 384–389, 1994.
19. O. Bruder, A. Wagner, M. Lombardi, J. Schwitter, A. van Rossum, G. Pilz, D. Nothnagel, H. Steen, S. Petersen, E. Nagel, S. Prasad, J. Schumm, S. Greulich, A. Cagnolo, P. Monney, C. C. Deluigi, T. Dill, H. Frank, G. Sabin, S. Schneider, and H. Mahrholdt, “European cardiovascular magnetic resonance (EuroCMR) registry — multi na-tional results from 57 centers in 15 countries,” J. Cardiovasc. Magn. Reson., vol. 15, no. 1, p. 9, Jan. 2013.
20. E. Reyes, C. Y. Loong, K. Latus, C. Anagnostopoulos, and S. R. Un-derwood, “Safety and tolerability of adenosine stress MPI in patients with asthma or chronic obstructive airways disease,” J. Nucl. Cardi-ol., vol. 11, no. 4, p. S5, 2004.
21. M. Motwani, N. Maredia, T. A. Fairbairn, S. Kozerke, A. Radjenovic, J. P. Greenwood, and S. Plein, “High-resolution versus standard-re-solution cardiovascular MR myocardial perfusion imaging for the de-tection of coronary artery disease.,” Circ. Cardiovasc. Imaging, vol. 5, no. 3, pp. 306–313, May 2012.
22. C. Manisty, D. P. Ripley, A. S. Herrey, G. Captur, T. C. Wong, S. E. Petersen, S. Plein, C. Peebles, E. B. Schelbert, J. P. Greenwood, and J. C. Moon, “Splenic Switch-off: A Tool to Assess Stress Adequacy in Adenosine Perfusion Cardiac MR Imaging,” Radiology, vol. 276, no. 3, pp. 732–740, Apr. 2015.
23. P. Kellman, M. S. Hansen, S. Nielles-Vallespin, J. Nickander, R. The-mudo, M. Ugander, and H. Xue, “Myocardial perfusion cardiovascu-lar magnetic resonance: optimized dual sequence and reconstruction for quantification,” J. Cardiovasc. Magn. Reson., vol. 19, no. 1, p. 43, 2017.
24. J. P. Greenwood, M. Motwani, N. Maredia, J. M. Brown, C. C. Eve-rett, J. Nixon, P. Bijsterveld, C. J. Dickinson, S. G. Ball, and S. Ple-in, “Comparison of cardiovascular magnetic resonance and single-photon emission computed tomography in women with suspected coronary artery disease from the Clinical Evaluation of Magnetic Resonance Imaging in Coronary Heart Disease (CE-MARC) Trial.,” Circulation, vol. 129, no. 10, pp. 1129–1138, Mar. 2014.
 This work is licensed under a
This work is licensed under a