Antoniu Octavian Petris1, Gabriel Tatu-Chitoiu2, Ioan Mircea Coman3, Diana Tint4, Ruxandra Christodorescu5, Valentin Chioncel6, Dan Darabantiu7, Diana Cimpoesu1, Laura Antohi8, Laurentiu Sorodoc1, Lucian Petrescu5, Calin Pop9, Alexandre Mebazaa10, Ovidiu Chioncel3
1 “Grigore T. Popa” University of Medicine and Pharmacy, “St. Spiridon” Emergency Clinical County Hospital, Iasi, Romania
2 Clinic of Cardiology, Emergency Clinical Hospital, Bucharest, Romania
3 “Carol Davila” University of Medicine and Pharmacy, “C.C. Iliescu” Emergency Institute for Cardiovascular Diseases, Bucharest, Romania
4 “Transilvania” University, Faculty of Medicine, ICCO Clinics, Brasov, Romania
5 “Victor Babes” University of Medicine and Pharmacy, Institute of Cardiovascular Medicine Timisoara, Romania
6 “Carol Davila” University of Medicine and Pharmacy, „Bagdasar Arseni” Emergency Clinical Hospital, Bucharest, Romania
7 “Vasile Goldis” University, Emergency County Hospital, Arad, Romania
8 “C.C. Iliescu” Emergency Institute for Cardiovascular Diseases, Bucharest, Romania
9 “Vasile Goldis” University, “Dr. Constantin Opris” Emergency County Hospital, Baia Mare, Romania
10 University Paris Diderot, Sorbonne Paris Cité, U942 Inserm, Hôpitaux Lariboisière „Saint Louis” University Hospitals, Paris, France
Abstract: Biomarkers have been accepted into daily clinical practice for several decades and are now widely used in the field of emergency cardiology, as a tool for quick diagnosis of some acute critical conditions requiring an early and adequate therapeutic approach. At fi rst, they were frowned upon (“guessing in the blood sample”) and then they were used indiscriminately (creating a new so-called disease – “troponinitis” etc). Utilization for diagnosis, risk stratifi cation or treatment strategy purposes requires an appropriate selection of the biomarkers, assuming specifi c variations in different clinical conditions. The development of these biomarkers have often caused another problem concerning the need for a more cautious interpretation, adapted to the type of patient with single-organ vs multi-organ failure who will require a quantitative multimarker high sensitivity approach: in this case point-of-care assessment is not enough. Reviewing the latest European Society of Cardiology (ESC) and European Resuscitation Council (ERC) Guidelines on acute cardio-vascular conditions (ESC Guidelines for STEMI– 2012, ESC Guidelines for acute pulmonary thromboembolism and Guidelines on the diagnosis and treatment of aortic diseases date from 2014, ESC Guidelines for NSTEMI and cardiac arrest from 2015 and ESC/HFA Guidelines for acute heart failure from 2016) we noticed the considerable proportion achieved by biomarkers as components of decision making in diagnostic, prognostic and therapeutic approaches. Some biomarkers are mentioned in almost all of these Guidelines (natriuretic proteins and cardiac troponins), while copeptin, H-FABP and GFD-15 are more and more frequently referenced in different acute critical conditions and, obviously, a number of particular biomarkers are specific only to certain acute situations (e.g. NSE, S100B in resuscitation, ST2, adrenomedullin and galactin-3 in heart failure). Clearly, it has lately become diffi cult to contest the role of biomarkers as biological signals that have to be taken into account in daily practice.
Keywords: biomarkers, emergency cardiology, resuscitation, acute coronary syndromes, pulmonary thromboembolism, acute heart failure, acute aortic syndromes
INTRODUCTION
Emergency medicine is an officially recognized medical specialty in many countries and is already pivotal in pre-hospital and Emergency Department settings. Chest pain, dyspnea, syncope and cardiac arrest are often reasons for hospitalization in the Cardiology Department or may occur during hospitalization. A signifi cant and delicate part of our daily activity in an ordinary cardiology clinic is dedicated to managing cardiovascular emergencies. Nowadays, biomarkers complement clinical assessment, 12-lead ECG and emergency imaging in the diagnosis, risk stratification and treatment of patients with acute cardiac syndromes1. Acute coronary syndromes (ACS), acute heart failure (AHF), acute pulmonary thromboembolism (PTE) and cardiac arrest are diagnoses that cause the most common challenges for any contact points with medical emergencies: pre-hospital, Emergency Department, Intensive Cardiac Care Units or cardiac wards. Of course, numerous cardiovascular biomarkers are now available even in primary care medicine. By adopting easy to use Point-of-Care (POC)-instruments, numerous false-positive ACS, AHF, and PTE diagnoses
were avoided, offering more appropriate ruling in/out arguments2. Moreover, biomarkers are mainly involved in cardiac arrests, alongside clinical examination, electrophysiology, and imaging, in a multimodal approach3 within the “fine art of prognostication”4. The notion of “biomarker”, already introduced in 1980, is considered to be, in the broad sense, “a characteristic that is objectively measured and evaluated as an indication of normal biologic processes, pathogenic processes, or pharmacologic responses to a therapeutic intervention”5 with the aim to improve prevention, prediction, diagnosis, and prognosis of cardiovascular disease and, last but not least, to decrease the related costs. Interest in using an improved diagnostic test in the emergency department and in the intensive cardiac care unit implies the need to reduce the use of hospital resources and associated costs using three main approaches: by providing arguments for early initiation of a highly effective therapy, thereby reducing the tendency of worsening patient clinical status and the development of complications, by eliminating the need for other, more expensive explorations, or by providing an alternative diagnosis allowing patient treatment in the Emergency Department or as an outpatient6. For instance, the use of a 3-in-1 point-of-care testing (POCT) for cardiac troponin T (cTnT), N-terminal pro-brain natriuretic peptide (NT-proBNP) and Ddimer in cardiovascular risk stratification at primary care level for diagnosing acute coronary syndromes (ACS), heart failure (HF) and thromboembolic events (TE) highlights the potential for substantial health-economic savings7. In a more strict sense, but adapted to current main research directions using improved automated analytical methodologies, “biomarkers” are those markers which are measured in biological specimens, such as cells or serum (e.g. microparticles, circulating micro- RNAs, blood transcriptomes, proteomics, metabolomics, lipidomics etc.)8, pieces of information “written in blood”9. A current selection of the most reliable biomarkers requires completion of 9-step criteria for their evaluation: 1. proof of concept, 2. prospective validation, 3. establishing their incremental value, 4. clinical usage, 5. clinical outcomes, 6. cost-effectiveness data, 7. ease of use, 8. methodological consensus and establishment of reference values (or, at least, cut-off values)10 (Table 1).
Biomarkers are used as a diagnostic tool according to specific rule-in/rule-out protocols that have been validated in clinical studies. On the one hand, the transfer of a potential reproducible, specific and sensitive biomarker from discovery to clinical practice (benchto-bedside) is not a simple process, mostly filled with pitfalls and limitations, which can be removed through a suite of strategies: improve the assay, combine several markers, check for subpopulations and stratify population11. On the other hand, in 2001, at a meeting of the American College of Cardiology, Robert Wilcox (quoted by Marc S. Sabatine)12 introduced (obvious ironically) new medical terms to which he added explanations:
– “Troponinite (tro’-po-nin-ite)13 n. a patient with low clinical probability of ischemia who is victimized by reflex responses to inconsequential borderline elevation of the cardiac troponins”.
– “Troponinister (tro’-po-nin-is’’-ter) n. a staunch (n.n. Wilcox use the term “rabid”), misguided advocate of the importance of troponins”.
Far from being seen at present as “icing on the cake”, increased biomarker levels must be interpreted only in a clinical context, otherwise, as it was bluntly put by DB Mark, “looking at biomarkers (A/N) in isolation may thus be akin to seeing smoke trailing out of the window of a house without having any notion of what is on fire, where that fire is, or how it can be extinguished”6. The progression of all of these biomarkers has often caused considerable diffi culties to the critical care physician who deals with multiorgan failure14. Some concentrations frequently lead to a more cautious interpretation and subsequent changes in therapeutic decisions rather than to the emergency physician who usually deals with patients presenting with chest pain, breathlessness or other single-organ pathology (Table2). Depending on whether biomarkers are organ specific vs. unspecific and disease specific vs. unspecific, their incremental clinical value (whether it is diagnostic, prognostic or serves to guide therapy) increases. Since these are components of patient management more and more frequently mentioned in daily practice, it would be useful to review the place of biomarkers in the current Guidelines on acute cardiac conditions (cardio-pulmonary resuscitation, acute coronary syndromes, pulmonary thromboembolism, and acute heart failure).
BIOMARKERS IN EMERGENCY CARDIOLOGY: CARDIAC ARREST AND CARDIO-PULMONARY RESUSCITATION
Biomarkers (cardiac, neurological and inflammatory) represent a growing area of interest in the heterogenous field of cardiac arrest, as they may provide the rescuers with early and valuable information on the severity of organ dysfunction in order for them to make a decision on clinical strategies and prognosticate outcomes17, even a decision on withdrawal of care, if this is admissible from a legal point of view. The most commonly used biomarkers to assess the degree of organ damage after cardiac arrest explore17: – the brain status (e.g. a neuronal isoform of the glycolytic enzyme enolase – NSE that is involved in glucose metabolism, an intracellular calciumbinding dimer implicated in neuronal differentiation and proliferation – protein S100B, the Glial Fibrillary Acidic Protein – GFAP, a monomeric intermediate-filament component of the astrocytic cytoskeleton, the Brain-Derived Neurotrophic Factor – BDNF, a brain glutamic oxalic transaminase, lactate dehydrogenase and lactate in the cerebrospinal fluid, Neural Cell Adhesion Molecule
– N-CAM, also called CD56 and selectins).
– the heart status (cardiac troponin – cTnI, cTnT and brain natriuretic peptide – BNP, creatine kinase – CK).
– the inflammation status that contributes to the development of exaggerate Systemic Inflammatory Response Syndrome – SIRS after cardiac arrest, so-called “sepsis-like syndrome”18, indirectly reflecting the peripheral circulation status as early as 3 hours after cardiac arrest (C-Reactive Protein
– hsCRP, Tumor Necrosis Factor – TNF-, Interleukin IL-1, -6, -8, procalcitonine – PCT, soluble Triggering Receptor Expressed on Myeloid cells 1 -sTREAM-1).
Plasma cytochrome c may have been studied in rats as a novel in vivo marker of mitochondrial injury after resuscitation from cardiac arrest that relates inversely with survival outcomes19, but this has not been proven in studies on human subjects20. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines for Postresuscitation Care (2015)21 stated that after cardiopulmonary resuscitation a multimodal approach to prognostication is essential and includes: clinical examination, electrophysiology, biomarkers and imaging. Cardiac biomarker testing (troponine and CK-MB) should quickly be evaluated in all patients who arrived/ developed a cardiac arrest in the Emergency Department associated with chest pain22. Cautionary, in case of cardiopulmonary resuscitation (CPR) two elements must be well-known23:
– the delay in release of biomarkers from damaged myocardium makes the certifi cation of acute myocardial infarction diagnosis difficult in the first hours after the onset of symptoms;
– the sensitivity and specifi city of the link between biomarkers and the acute coronary artery occlusion as the cause of cardiac arrest are low, as many other factors can be involved: myocardial injury during resuscitation maneuvers and defibrillation, the duration of resuscitation or of cardiac arrest with diffuse myocardial hypoperfusion, especially if a coronary artery disease is present in the patient’s history.
Figure 1. Biomarkers in emergency cardiology: a synopsis from current ESC/ERC Guidelines. ACS=acute coronary syndrome; AHF=acute heart failure; PT=pulmonary Thromboembolism; CA=cardiac arrest; CPR=cardio-pulmonary resuscitation. cTn=cardiac troponin; NPs=natriuretic peptides; NSE=neuronal enolase; PCT=procalcitonin; H-FABP=heart-type fatty acid-binding protein; 15 GFD=growth differentiation factor; MMP=matrix metalloproteinase; selastin= soluble elastin fragments; B-TGF=transforming growth factor beta; sST2=soluble suppresion of tumorigenicity 2; Gal-3=galactin 3. *biomarkers tested but not yet used into clinical practice.
The values of hs-TnT. even if they are elevated in patients with out-of-hospital cardiac arrest ventricular fibrillation or tachycardia, do not improve risk prediction either, as showed by the data from the prospective FINNRESUSCI study24. It was believed that percutaneous coronary interventions (PCI), especially if performed early after hospital admission, could influence troponin levels after a cardiac arrest/CPR, but in a small study PCI did not significantly alter serum levels of cardiac markers (cTnI) in patients with AMI compared with those with non-ST elevation CA25. The predictive value of NT-pro-BNP/BNP in cardiac arrest/CPR is altered by many factors (myocardial stretching, chronic obstructive pulmonary disease, pulmonary thromboembolism, thyroid disease, sepsis, renal failure), but high BNP levels have been associated to poor outcomes without knowing yet exactly if elevated levels refl ect the presence of an significant cardiac overload after global ischemia or just quantify the extent of brain damage26. NSE and S-100B are protein biomarkers released following injury to neurons and glial cells, respectively, that are likely to correlate with the extent of anoxic– ischaemic neurological injury and with the severity of neurological outcomes, but hemolysis in blood samples may lead to a potential misclassification prognosis for NSE levels and will not affect S100B values17,27. The advantages of the two already mentioned neurological biomarkers compared to both electroencephalography and clinical examination are that they provide quantitative results and they are sedatives effects free. It should be noted, however, that only the combination of these biomarkers with other diagnostic tools (e.g., SSEP, EEG, MRI and clinical examination) could significantly improve their sensitivity and specificity in predicting outcomes after cardiac arrest/ CPR and none of these biomarkers can predict a good cerebral post CPR outcome17. In addition, it is difficult to find a cut-off value accurate enough to identify patients with a poor outcome with a high degree of certainty and the NSE levels may be significantly reduced during therapeutic hypothermia28. Hence, NSE above 28 microg/l at 48 h and a rise in NSE of more than 2 microg/l between 24 and 48 h were markers for a poor outcome after cardiac arrest and induced hypothermia29. The combination of brain CT and serum NSE improves the prognostic performance when compared to either alone in predicting poor neurologic outcome in cardiac arrest patients treated with therapeutic hypothermia30. Increasing NSE levels are more suitable than its absolute serum levels for the prediction of poor neurologic outcomes31. Biomarkers, Somatosensory Evoked Potentials (SSEP) and imaging studies may play a prognostication role in patients with prolonged sedation and/or paralysis, since they are insensitive to drug interference22. Only procalcitonin (PCT) as biomarker of infl ammation seems to have some correlation with poor outcomes, but, as with other inflammatory markers, sepsis acts as camouflage in the prognostication of patients with hypoxic encephalopathy32. Circulating microRNAs (miRNAs), non-protein coding RNA molecules that are evolutionarily conserved and ubiquitously expressed as regulators of gene expression, are released very early after cardiac injury and may be useful predictors of neurological outcomes and survival after cardiac arrest33.
BIOMARKERS IN EMERGENCY CARDIOLOGY: ACUTE CORONARY SYNDROMES
Along with acute dyspnea and often associated to this, acute chest pain is one of the most common emergency complaints: around 10 million patients per year in Europe and 8 million in the US34,35. Therefore, errors can occur: on the one hand, only approximately onethird of patients are diagnosed with an ACS, on the other hand, among patients who are diagnosed with non-cardiac chest pain, 1-4% actually have ACS, making these missed diagnoses a reason for increased mortality36.
ST elevation myocardial infarction (STEMI)
Nowadays, biomarkers make the distinction between the occurrence of just ischemia or necrosis in acute coronary syndromes, and therefore the current international consensus definition (“universal definition of myocardial infarction”) states that the term “acute myocardial infarction” should be used only when there is evidence of myocardial necrosis in a clinical setting where myocardial ischaemia occurred37. As set forth by all current guidelines, a combination of criteria is required to meet the diagnosis of acute myocardial infarction”. The detection of rise and/or fall of cardiac biomarker values (preferably any member of the troponin familly: hs+/- T, I,) with at least one value above the 99th percentile of the upper reference limit is essential, associated with at least one of the following38:
• symptoms of ischaemia;
• new or presumably new signifi cant ST-T changes or new LBBB;
• development of pathological Q waves in the ECG;
• imaging evidence (usual echocardiographic) of new loss of viable myocardium, or new regional wall motion abnormality;
• identification of an intracoronary thrombus via angiography or autopsy.
Cardiac biomarkers should be measured in all patients who present with chest discomfort consistent with ACS (Level of Evidence: B)39. Blood sampling for serum markers is routinely carried out in the acute phase, but one should not wait for the results before initiating reperfusion treatment. Troponin (T or I) is the biomarker of choice, with a proven higher sensitivity and specificity for cardiomyocyte injury than creatine kinase (CK), its MB isoenzyme (CK-MB) and myoglobin8. The development of high-sensitivity (hs) assays for troponin I and T has improved the diagnostic sensitivity for acute myocardial infarction, decreased the time to diagnosis (cardiac troponins rise rapidly, usually within 1 h after symptom onset, but remain still elevated for a variable period of time, usually several days) and led to quicker rule-out of myocardial ischaemia8. High-sensitivity assays allow overcoming the sensitivity-deficit of the cTn assays available at that time resulting in the “troponin-blind period” and are recommended over less sensitive ones. In a study that revealed correlation between the angiographic culprit lesions and cardiac biomarkers hs-TnT had the highest sensitivity for prediction of lesions requiring emergency PCI and heart-type fatty acid-binding protein (HFABP) displayed significant correlations with a number of diseased vessels and the presence of a thrombotic lesion40. CK-MB shows a more rapid decline after acute myocardial infarction (CKMB is released within 2-4 hours, peaks at 24 hours following pressure overload or ischemia, and returns to normal by 36-72 hours) as compared with cardiac troponin (an increase occurring 2-4 hours after symptoms and remaining elevated for 7-14 days)41 and may provide added value for the timing of myocardial injury and the detection of early reinfarction14. The assessment of copeptin, the C-terminal part of the vasopressin prohormone, may quantify the endogenous stress level in multiple medical conditions (e. g. cardiac ischemia). Copeptin may be used in addition to high-sensitivity cardiac troponin for the early rule-out of MI42. Natriuretic peptides rise in response to increased myocardial wall stress14. The precursor peptide of BNP stored in granules of ventricular myocytes is cleaved in response to wall stress and myocyte stretch into its physiologically active hormone, B-type natriuretic peptide (BNP) and N-terminal pro-BNP – BNP, both being released simultaneously into the serum as result of increasing hemodynamic stress and cardiac ischemia43. However, many studies have established the role of natriuretic peptides in diagnosing, staging, making admission/discharge decisions and identifying patients at risk for adverse clinical events in various acute critical conditions. Normal levels have robust negative predictive value. There are certain limitations in using these biomarkers. Although studies did not meet sensitivity/specificity criteria for the use of BNP or NT-proBNP in the diagnosis of ACS, they supported the addition of BNP as part of a multimarker approach with TnI, CK-MB and myoglobin to increase the sensitivity of diagnosing AMI from 86% to 100% in chest pain patients presenting to the emergency department without any clinical signs of congestive heart failure43. Associated clinical conditions such as advanced age, obesity, tachyarrhythmia, LV hypertrophy, renal dysfunction and some therapeutic resources may influence NPs levels. Even though for acute heart failure some limits are set, there are no defi nitive cut-off values in patients with signs and symptoms of heart failure following acute myocardial infarction39. Heart Fatty Acid-Binding Protein (H-FABPs) are small cytosolic proteins that are important in the transport of long-chain fatty acids in cardiac myocytes. After cardiac ischemia and subsequent cell membrane damage, H-FABP is released into the extracellular space within 1-3 hours, returning to normal within 12-24 h14,43. The diagnostic sensitivity of H-FABP for cardiac injury is 93.1%, higher than CK-MB, cTn and myoglobin for the early diagnosis of AMI within fi rst 6 hours of chest pain44,45. H-FABP has shown no additional diagnostic value in patients suspected of acute coronary syndrome presenting to the emergency department when hscTnT measurements are also available46. The Matrix MetalloProteinase (MMPs) family consists of enzymes involved in extracellular matrix degradation47. In the setting of myocardial infarction, coronary mast cells accumulate at the site of an eroded or ruptured plaque as part of an inflammatory response and release proteases that degrade the extracellular matrix via activation of MMPs, particularly MMP-9. MMP-9 is considered a proximal biomarker (shows a close relationship with its target disease) for cardiac remodeling and a distal biomarker (exhibits non-targeted disease modifying outcomes) for inflammation48. Higher levels of Growth Differentiation Factor-15 (GDF-15) in patients with ACS are associated with raised risks of all types of major non-CABG-related bleeding, spontaneous MI and stroke, as well as CV disease and total mortality beyond established risk factors, 1 SD increase in ln GDF-15 being associated with increased risk of major bleeding and with a similar increase in risk across different bleeding locations49.
Non-ST-Segment Elevation ACS (Non-STE-ACS)
Although the names of the two clinical Non-STE-ACS entities include an ECG pattern, at the myocardial level cardiomyocyte necroses express themselves through biomarker release: NSTE-myocardial infarction (NSTEMI) and myocardial ischaemia without cell loss (unstable angina – UA)50. Biomarkers (mainly troponin) join clinical assessment and 12-lead ECG (the right and posterior lead can also be helpful) in the diagnosis, risk stratifi cation and treatment of patients with suspected Non-STEACS38. In the last ACCF/AHA Guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction51 once it has been established that no biomarker of myocardial necrosis has been released (based on 2 or more samples collected at least 6 h apart, with a reference limit of the 99th percentile of the normal population), the patient with ACS may be considered to have experienced unstable angina (UA), whereas the diagnosis of NSTEMI is established if a biomarker has been released. The UA Guidelines affirm, with a Level of Evidence B, that, taking into account the uncertainties often present with the exact timing of onset of pain and the sensitivity, precision, and institutional norms of the assay being utilized, as well as the release kinetics of the biomarker being measured, patients with negative cardiac biomarkers within 6 h of the onset of symptoms consistent with ACS should have biomarkers re-measured in the time frame of 8 to 12 h after symptom onset51. NSTEMI can already be ruled-out upon presentation, if the hs-cTn concentration is very low. NSTEMI can also be ruled-out by the combination of low baseline levels and the lack of a relevant increase within 1 h / 3 h (“rule-in” and “rule-out’ algorithms). 0 h and 1 h/3h refer to the time from the fi rst blood test50. “0 h/1 h algorithm” when high-sensitivity cardiac troponin assays are available is based on two concepts52:
– hs-cTn is a continuous variable and the probability of acute myocardial infarction increases with increasing hs-cTn values;
– early absolute changes of the levels within 1 h can be used as surrogates for absolute changes over 3 h or 6 h.
Cut-off levels are assay-specific. Recently, C. Műller highlighted seven clinical rules that may help to ensure best-contemporary clinical use of hs-cTn blood concentrations in the early diagnosis of acute myocardial infarction39:
1. First, do not forget the patient! Early diagnosis is based on the careful integration of all information derived from detailed clinical assessment, ECG patterns and the blood concentration of cardiac troponin (cTnT or cTnI).
2. Do not forget the ST-segment elevation! Both hscTn 0 h/3 h or 0 h/1 h-algorithms are applied after the initial 12-lead ECG has ruled-out the presence of signifi cant ST-segment elevation, which should trigger emergency myocardial revascularization.
3. Do not forget the pre-test-probability! Do not measure cTn in critically ill patients in the intensive cardiac care unit, unless there is a high pre-test-probability for acute myocardial infarction, because many other causes of cardiomyocyte damage can generate very lowpositive predictive values of elevated hs-cTn blood concentrations.
4. Do not forget the staff training! The proper education and training of nurses and juniors doctors avoid diffi culties in implementing the hs-cTn assay and/or the hs-cTn 0 h/1 h-algorithm.
5. Keep calm and carry on! hs-cTn assays allow for a shorter time interval to the second cTn measurement after 1, 2, or 3 h reducing time to diagnosis and/ or right management, stay and costs in the Emergency Department.
6. Being troponin-positive is not enough! hs-cTn is a quantitative marker of cardiomyocyte injury, the higher its blood concentration, the higher the likelihood for acute myocardial infarction and vice versa. Moreover, very low hs-cTn concentrations at presentation have shown a very high negative predictive value for acute myocardial infarction and were associated with extremely low mortality rates at 30 days.
7. Nothing is 100%! Both the hs-cTn 0 h/3 h- and 0 h/1 h-algorithms allow for a safe and early triage of patients, but do not allow for a 100% diagnosis label. Coronary angiography should be considered in patients for whom there is a high degree of clinical suspicion of N-STE-ACS, while in patients with low to intermediate likelihood for this condition, computed tomography (CT) coronary angiography is a solution to keep in mind.
A useful piece of information is that high-sensitivity cardiac troponin assays also maintain high diagnostic accuracy in patients with renal dysfunction, but “assayspecific” optimal cut-off levels, which are higher in patients with renal dysfunction, should be used54. Multiple biomarkers have been associated with mortality in NSTE-ACS: the natriuretic peptides (i.e. B-type natriuretic peptide, N-terminal pro-B-type natriuretic peptide and midregional pro-A-type natriuretic peptide) provide prognostic information on top of cardiac troponin, the high-sensitivity C-reactive protein and novel biomarkers such as midregional proadrenomedullin (MPAM), growth differentiation factor 15 and copeptin55. However, because these biomarkers have not shown to improve patient management or to increase additionally the short and mid-term prognostic, their routine use cannot be recommended by the Guidelines in the meantime. Large cohort studies have shown that, even in the range of “normal values”, detectable levels of hs- cardiac troponin I identify individuals with cardiac risk for adverse cardiovascular events, even if not diagnosed with acute myocardial infarction, as this may reflect myocite stress56. Therefore, hs-cardiac troponins, may lack specificity, but the sensibility is critical important, when used for the diagnosis of acute myocardial infarction. At this point, the most accurate test that can be used for the diagnosis of acute myocardial infarction remains the assessment of cardiac troponins57.
Current guidelines make recommendations over the use and interpretation for each assay in particular58. At the same time, hs-troponins and troponins allow a good negative predictive value, when adequately used (different time frame strategy: 2 test at 1-3 hours vs. 3 tests at 6 hours).
BIOMARKERS IN EMERGENCY CARDIOLOGY: BIOMARKERS IN ACUTE AORTIC SYNDROMES
Aortic dissection also involves an inflammatory response that precludes the dissection, but having a role in the destruction of the median tunic and which is further increased after it occured59. Once aortic dissection has occured, there is injury and destruction of the smooth muscle cells which release into the blood fl ow preoteolysis products and, on the other hand, there are lytic processes of the thrombus in the false lumen59. The most important markers used in patients with aortic syndromes are D-dimers. D-dimers (consist of two cross-linked D fragments from fibrinogen) are produced as a consequence of the activation of coagulation when generation followed by degradation of cross-linked fi brin take place60. But as there is a close connection between inflammation and coagulation, Ddimers are not just markers of fi brinolysis60. Also the values are expected to be increased in the elderly and women61. Their levels increase rapidly, up to one hour, adding great utility to fast diagnosis59,62. But negative D-Dimers (using the same cut-off value as in pulmonary embolism – 500 ug/l, while having higher negative predictive value for values <100 ug/l) should only rule out an aortic syndrome, in those patients with a low clinical probability; they have no incremental diagnostic value when high risk patients are evaluated62,63. Also D-Dimers should not be used in pregnancy, malignancy, infections, post-surgery, trauma (<4 weeks) or liver cirrhosis as they lack specifi city60. There is data on the prognostic value in patients that have been treated with endovascular aortic repair as high persistent values (more than 20 days) have been associated with increased mortality59. However, It should be noticed, that the different assays for the detection of D-Dimers are not well standardized and they should be interpreted with caution, according to clinical practice guidelines59,60,64. Several other biomarkers have been investigated for making an early diagnosis, for the monitoring and establishing the prognosis: – markers of the destruction of smooth muscle cells (smooth muscle myosin) or the the vascular interstitium (calponin, matrix metalloproteinase 8); – structural proteins of the aorta – soluble fragments of elastin (although its levels increase in less than an hour, the abnormal values are close to the normal interval);
– inflammation markers – C reactive protein (its peak level correlates with a tendency to increased remodelling), tenascin-C;
– circulation beta-TGF59,65.
BIOMARKERS IN EMERGENCY CARDIOLOGY: PULMONARY THROMBOEMBOLISM (PTE)
Right ventricular pressure overload is associated with increased myocardial stretch, which leads to the release of brain natriuretic peptide (BNP) or N-terminal (NT)-proBNP – markers of right ventricular dysfunction66. Markers of right ventricular (RV) dysfunction and the markers of myocardial injury are utilized in the classification of patients with acute PTE based on early
mortality risk66:
– patients with RV dysfunction (on echocardiography or CT angiography) and elevated cardiac biomarker levels (particularly a positive cTn test) should be classifi ed into an intermediate-highrisk category;
– patients in whom the RV is normal (on echocardiography or CT angiography), and/or have normal cardiac biomarker levels, belong to an intermediate- low-risk group.
In patients at intermediate risk, that is the risk category most diffi cult to characterize in terms of prognosis, assessment of the right ventricle using echocardiography or CT and of myocardial injury using a laboratory biomarker, should be considered for further risk stratification, finding quoted in the last Guidelines for diagnosis and treatment of PTE in the class of recommendation IIa, level of evidence B67. A meta-analysis found that 51% of 1132 unselected patients with acute PTE had elevated BNP or NTproBNP concentrations on admission, which is associated with a 10% risk of early death and a 23% risk of an adverse clinical outcome68. However, in normotensive patients with PTE, the positive predictive value of elevated BNP or NT-proBNP concentrations for early mortality is low69, and suggesting that higher cut-off values should be considered. In a prospective, multicentre cohort study that included 688 patients, using a stepwise approach based on the simplifi ed Pulmonary Embolism Severity Index (PESI score), NT-proBNP ≥600 pg/mL was identifi ed as the optimal cut-off value for the identification of elevated risk70. Elevated plasma troponin concentrations on admission of patients with acute PTE have been reported in connection with PE and were associated with worse prognosis. A meta-analysis covering a total of 1985 patients showed elevated cardiac troponin I or T concentrations in approximately 50% of the patients with acute PTE, results consistent for troponin I or T and for prospective or retrospective studies71. Some studies have found that elevated troponin concentrations were associated with high mortality both in unselected patients and in haemodynamically stable patients; however, other systematic reviews and meta-analyses of troponin-based risk stratifi cation of normotensive patients with acute symptomatic PTE have suggested a limited prognostic value of elevated troponins in normotensive patients72. In a prospective, multicentre cohort of 526 normotensive patients with acute PTE, troponin T concentrations <14 pg/mL, measured by a high-sensitivity assay, had a negative predictive value of 98% with regard to a complicated clinical course, which was similar to that of the sPESI, combination of cTnI – sPESI being able to identify possible candidates for out-of-hospital treatment73. Similarly, the value of NT-proBNP <500 pg/mL as a laboratory biomarker for selecting candidates for home treatment with clinically defi ned very low-risk PTE revealed that approximately 45% of patients with PTE can be treated in an outpatient setting. None of patients died or suffered recurrence of VTE or major bleeding complications during the three-month follow-up, and there was no increase in patient anxiety scores74. In a meta-analysis that has reviewed the role of biomarkers such as B-type natriuretic peptides (BNP and NT-proBNP) and troponins in risk stratification of acute PTE, BNP appeared to have better sensitivity and specifi city than NT-proBNP in detecting right ventricular dysfunction. Raised levels of B-type natriuretic peptides at admission identifi ed a subset of patients at higher risk of adverse outcomes and among patients with raised natriuretic peptide levels, increased troponins were found to be an independent prognostic biomarker75. Heart-type fatty acid-binding protein (H-FABP), an early marker of myocardial injury, was also found to significantly predict mortality in patients with acute PTE at intermediate risk. It is signifi cantly associated with impaired right ventricular function and shows better correlation with mortality than troponin I76. H-FABP might be a useful biomarker for risk stratifi cation of normotensive patients with acute PTE where circulating H-FABP levels ≥6 ng/mL had a positive predictive value of 28% and a negative predictive value of 99% for an adverse 30-day outcome77. A simple score for immediate risk stratifi cation of non-high-risk patients, based on the presence of tachycardia, syncope, and a positive bedside test for H-FABP (a positive result for plasma concentration >7 ng/ml), provided prognostic information similar to that of RV dysfunction on echocardiography78. The FAST score prognostic score (H-FABP, Syncope, and Tachycardia; FAST score) where the determination of H-FABP by immunoturbidimetry provides prognostic information superior to that of ELISA, appears more suitable to identify patients with an adverse 30-day outcome compared to the ESC Guidelines model and sPESI79. A meta-analysis of studies in patients with acute PTE for the purpose of identifying the prognostic value of elevated D-dimer levels for short-term (within 30 days) and 3-month mortality has shown that elevated D-dimer concentrations were associated with increased short-term mortality in some studies, while levels <1500 ng/mL had a negative predictive value of 99% for excluding three-month all-cause mortality80. Searching in 13 databases (n = 1585 patients), the Cochrane Report emphasizes that the negative D-dimer test is valuable in ruling out PTE in patients who present in an emergency setting with a low pre-test probability of PTE determined according to a clinical prediction rule. This test may probably be less useful in older populations (high levels of false-positive results), but no empirical evidence was available to support an increase in the diagnostic threshold of interpretation of D-dimer results for patients over the age of 6581. In summary, it is recommended to test D-dimers only in non-high risk suspected pulmonary embolism, when the clinical probability is low or intermediate, with the purpose to exclude the disease in the emergency department and thus reduce the use of unnecessary irradiation imaging; preferably a highly sensitive assay should be used. The use of the different commercial assays should take note that not all are clinically validated by studies60. Growth differentiation factor (GDF)-15, a cytokine induced in the heart after ischemia or pressure overload has elevated levels on admission in patients with acute PTE and was strongly and independently related to an increased risk of death or major complications during the first 30 days after diagnosis, prognostic in formation additive to that of the established biomarkers cTnT and NT-proBNP, and to echocardiographic findings of right ventricular dysfunction82. Biomarkers that usually grow in renal injuries were found related to 30-day all-cause mortality in acute PTE: elevated serum creatinine levels, a decreased (calculated) glomerular fi ltration rate, elevated neutrophil gelatinase-associated lipocalin (NGAL) and cystatin C (N-GAL >75 ng/ml and cystatin C >1900 ng/ml). On the other hand, impaired kidney function was found present in 47% of acute PTE patients83,84. Multimarker models integrating information obtained from echocardiography (evidence or exclusion of RV dysfunction) in combination with some laboratory biomarkers (mainly BNP/NT-proBNP, cTnT/hsTnT, GDF-15 or H-FABP) have been reported to improve risk stratification of acute PTE, but require the prospective confirmation of the large studies. Biochemical markers (for vascular dysfunction, inflammation, myocardial stress, low cardiac output, organ damage) have also been investigated for pulmonary hypertension (PHT), but without any significant clinical value regarding specific diagnosis. The current pulmonary hypertension guidelines only mention natriuretic peptides for their use in the prognostic evaluation at the time of diagnosis and during follow-up (and regarding response to therapy) in PHT patients. BNP values above 300 ng/l and Nt-proBNP values above 1400 ng/l seem to identify a high risk population, when integrated into an algorithm along with other clinical and echocardiographic parametres85.
BIOMARKERS IN EMERGENCY CARDIOLOGY: ACUTE HEART FAILURE (AHF)
Although the subject of great interest in recent years, the current Guidelines (2016) for diagnosis and treatment of heart failure has few references to the use of biomarkers in acute heart failure: upon presentation to the ED or CCU/ICU, a plasma NPs level (BNP, NT-proBNP or MR-proANP) should be measured in all patients with acute dyspnea and suspected AHF to help in the differentiation of AHF from non-cardiac causes of acute dyspnea86. NPs have high sensitivity, and normal levels in patients with suspected AHF make the diagnosis unlikely. Elevated levels, as recommended by ESC/HFA guidelines, in the non-acute setting are BNP >35 pg/ml and/ or NT-proBNP >125 pg/mL. In the acute setting, higher values should be used: BNP >100 pg/mL, NT-proBNP >300 pg/ mL and mid-regional pro A-type natriuretic peptide (MR-proANP) >120 pmol/L86. Although there is extensive research on biomarkers in HF (e.g. soluble ST2, galectin 3, copeptin), there is no defi nite evidence to recommend them for clinical practice87,88. One must always bear in mind that elevated levels of NPs do not automatically confirm the diagnosis of
AHF, as they may also be associated with a wide variety of cardiac and non-cardiac causes. On the other hand, low levels of NPs can be detected in some patients with decompensated end-stage
HF, fl ash pulmonary edema or right sided AHF. Mid-regional proADM (adrenomedullin MRproADM) is released from a multitude of tissues, and also has potent vasodilatory, hypotensive, and natriuretic effects. A large (1.641 patients presenting to the emergency department with dyspnea), multicenter prospective study demonstrated noninferiority of MRproANP to BNP for the diagnosis or exclusion of acute HF in patients presenting to the Emergency Department with dyspnea. Moreover, this study found that MR-proADM was superior to BNP or NT-proBNP in identifying dyspneic patients with acute decompensated HF at high risk of 90-day mortality89. Detection of ACS as the underlying cause of AHF requires the quantifi cation of cardiac troponins’ level; this is particularly relevant, as large studies have shown that when an acute coronary syndrome is the precipitating factor in acute heart failure, the mortality rate is higher90. Elevated concentrations of circulating cardiac troponins are frequently detected in patients with AHF, often without obvious myocardial ischaemia or an acute coronary event, or without coronary artery
stenoses and are associated with worse outcomes90,91. Initial blood pressure and troponin I, can help identify patients with congestive heart failure at low risk for prolonged hospitalization and adverse events since the Emergency Department92. Mid-regional proANP (MR-proANP) is released from the atria in response to increased atrial stretch, and has diuretic, natriuretic, and vasodilatory effects93. Galectin 3 (Gal-3), a member of the -galactosidebinding lectin family, is associated with adverse remodeling via activated cardiac fi broblasts and macrophages94.
The prognostic value of Gal-3 is additive with NPs levels and Gal-3 are independently predictive of recurrent decompensations and death in patients with heart failure95. American Heart Association/American College of Cardiology Foundation heart failure Guidelines consider a class IIB indication for the use of a Gal-3 assay for additive risk stratifi cation in patients with established heart failure96. A study (4964 patients) found elevations in Gal-3 correlated with an increased risk of heart failure and cardiovascular death in patients after ACS, indicates a
potential role for Gal-3 in monitoring patients after ACS97. Soluble suppression of tumorigenicity 2 (sST2), a member of the IL-1 receptor family, secreted into the circulation by cardiomyocytes and pulmonary endothelial cells, inhibits IL-33 and, through this, is downregulating the infl ammatory response98. High levels of sST2 correlate with disease severity and increased morbidity in patients with ADHF and ACS, providing complementary prognostic information to hs-cTnT and NT-proBNP99. ESC/HFA Guidelines 2016 indicates that the assessment of procalcitonin levels may be useful in patients with AHF with suspected coexisting infection, particularly for the differential diagnosis of pneumonia and to guide antibiotic therapy. The results from the BACH trial emphasize that patients with a diagnosis of AHF and an elevated PCT concentration (>0.21 ng/mL) had a worse outcome if not treated with antibiotics, while patients with low PCT values (<0.05 ng/mL) had a better outcome if they did not receive antibiotic therapy100. Multiple other biomarkers, including those reflecting inflammation, myocyte oxidative stress, neurohormonal disturbances, matrix remodelling, fi brosis, (most biomarkers integrate information from different disease pathways, e.g. Gal-3 is thought to represent a “link” between inflammation and fibrosis), extracardiac dysfunction, such as acute kidney injury, have been investigated for their diagnostic and prognostic value in AHF and some are developed as “companion biomarkers” to identify patients with the greatest benefit from a therapeutic intervention. Even though none of them has reached the stage of being recommended for routine clinical use, plasma biomarkers, along with imaging and genetic testing, might be used to define HF subtypes responding differently to specific therapeutic interventions101. A differential predictive value in HF reduced ejection fraction vs. HF preserved ejection fraction was suggested for some biomarkers, as they assess different pathophysiological pathways102.
At this point, biomarkers are being studied not just for their diagnostic value, but also for the prognostic value at admission (with the aim to identify the patients in need of more intensive care), or at discharge (to identify patients at risk for early postdicharge events) (Table 3). But the predictive value of different biomarkers diminishes with time, and multimarker strategy may be more useful102,103,104. By extensively stretching the diagnosis palette, into complex and high-risk patients with AHF, for instance, it is possible to use biologically “orthogonal” markers (a multimarker approach) such as NT-proBNP (stress), sST2 (myocardial fibrosis/remodeling), highly sensitive troponin (myocardial injury), MR-proADM (hemodynamic stress), copeptin (salt/water derangement), and renal biomarkers105. The “fi ne tunning” approach, in the use of these biomarkers for diagnosis, risk stratification or treatment strategy purposes requires a good selection of them and a check on their variations in various clinical conditions of emergency cardiology (Figure 1)106.
CONCLUSIONS
Reviewing the latest Guidelines on acute clinical conditions that can be encountered in the field of emergency cardiology we noted the increasing importance achieved by biomarkers as components of diagnostic approache, prognostic stratifi cation and adequate management. However, biomarkers should not be used as an alternative to clinical judgement or to other validated
imaging tools. During decision-making process in different clinical settings, judicious use of biomarkers may expedite early diagnosis and may improve early triage and management strategies, but always in conjunction to the well established clinical algorithms. Further research will be needed to validate biomarkers as part of multimarker strategy in different clinical conditions.
Author contributions: All authors contributed to this manuscript.
Supported by: none.
Conflict-of-interest: none declared.
The manuscript represents the conclusions of the Consensus Meeting “Biomarkers in Emergency Department”, coordinated by Alexandre Mebazaa and Ovidiu Chioncel, Sinaia, September 2016, and reflects the official position of the Acute Cardiac Care and Heart Failure Working Groups of the Romanian Society of Cardiology.
Acknowledgements: The authors thank Călin Pop for English text revision.
References
1. Jaffe AS, Babuin L, Apple FS: Biomarkers in acute cardiac disease: the present and the future. J Am Coll Cardiol 2006, 48: 1-11.
2. Tomonaga Y, Gutzwiller F, Lüscher TF, Riesen WF, Hug M, Diemand A, Schwenkglenks M, Szucs TD. Diagnostic accuracy of point-of-care testing for acute coronary syndromes, heart failure and thromboembolic events in primary care: a cluster-randomised controlled trial. BMC Fam Pract 2011; 12: 12-20.
3. Nolan JP, Soar J, Cariou A, Cronberg T, Moulaert VR, Deakin CD, Bottiger BW, Friberg H, Sunde K, Sandroni C. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines for Post-resuscitation Care 2015: Section 5 of the European Resuscitation Council Guidelines for Resuscitation 2015. Resuscitation 2015; 95: 202-22.
4. Cowie MR. The fine art of prognostication. Eur Heart J 2002; 23: 1804–1806.
5. Biomarkers Defi nitions Working Group. Biomarkers and surrogate endpoints: preferred defi nitions and conceptual framework. Clin Pharmacol Ther 2001; 69: 89–95.
6. Mark DB, Felker GM. B-type natriuretic peptide – a biomarker for all seasons? N Engl J Med 2004; 350: 718-20.
7. Tomonaga Y, Gutzwiller F, Lüscher TF, Riesen WF, Hug M, Diemand A, Schwenkglenks M, Szucs TD. Diagnostic accuracy of point-of-care testing for acute coronary syndromes, heart failure and thromboembolic events in primary care: a cluster-randomised controlled trial. BMC Fam Pract 2011; 12: 12-20.
8. Hoefer IE, Steffens S, Ala-Korpela M, Bäck M, Badimon L, Bochaton- Piallat ML, Boulanger CM, Caligiuri G, Dimmeler S, Egido J, Evans PC, Guzik T, Kwak BR, Landmesser U, Mayr M, Monaco C, Pasterkamp G, Tuñón J, Weber C; ESC Working Group Atherosclerosis and Vascular Biology. Novel methodologies for biomarker discovery in atherosclerosis. Eur Heart J 2015; 36: 2635-42.
9. Liotta LA, Ferrari M, Petricoin E. Clinical proteomics: written in blood. Nature 2003; 425: 905.
10. Vlachopoulos Ch, Xaplanteris P, Aboyans V, Brodmann M, Cífková R, Cosentino F, De Carlo M, Gallino A, Landmesser U, Laurent S, Lekakis J, Mikhailidis DP, Naka KK, Protogerou AD, Rizzoni D, Schmidt- Trucksäss A, Van Bortel L, Weber T, Yamashina A, Zimlichman R. The role of vascular biomarkers for primary and secondary prevention. A position paper from the European Society of Cardiology Working Group on peripheral circulation Endorsed by the Association for Research into Arterial Structure and Physiology (ARTERY) Society. Atherosclerosis 2015; 241: 507-532.
11. Drucker E, Krapfenbauer K. Pitfalls and limitations in translation from biomarker discovery to clinical utility in predictive and personalised medicine. EPMA J 2013; 4: 7-16.
12. Sabatine MS. Biomarkers in Acute Coronary Syndromes. FAID 2007. Available from: URL: http://faid2007.france-science.org/presentations/ Sabatine.pdf
13. Kramer CM. Avoiding the imminent plague of troponinitis: the need for reference limits for high-sensitivity cardiac troponin T. J Am Coll Cardiol 2014; 63: 1449-50.
14. McLean AS, Huang SJ. Cardiac biomarkers in the intensive care unit. Ann Intensive Care 2012; 2: 8-18.
15. Gupta DK, Wang TJ. Natriuretic peptides and cardiometabolic health. Circ J 2015; 79: 1647–1655.
16. Zois NE, Bartels ED, Hunter I, Kousholt BS, Olsen LH, Goetze JP. Natriuretic peptides in cardiometabolic regulation and disease. Nat Rev Cardiol 2014; 11: 403–412.
17. Scolletta S, Donadello K, Santonocito C, Franchi F, Taccone FS. Biomarkers as Predictors of Outcome After Cardiac Arrest. Expert Rev Clin Pharmacol 2012; 5: 687-699.
18. Adrie C, Adib-Conquy M, Laurent I, Monchi M, Vinsonneau C, Fitting C, Fraisse F, Dinh-Xuan AT, Carli P, Spaulding C, Dhainaut JF, Cavaillon JM. Successful cardiopulmonary resuscitation after cardiac arrest as a “sepsis-like” syndrome. Circulation 2002; 106: 562-8.
19. Radhakrishnan J, Wang S, Ayoub IM, Kolarova JD, Levine RF, Gazmuri RJ. Circulating levels of cytochrome c after resuscitation from cardiac arrest: a marker of mitochondrial injury and predictor of survival. Am J Physiol Heart Circ Physiol 2007; 292: H767-75.
20. Petriş A, Ungureanu D, Popa TO, Costache I, Cimpoeşu. Evaluation of biomarkers and use of echocardiography in survival prognosis post cardio-respiratory arrest. Resuscitation 2015; 96: 148-149.
21. Nolan JP, Soar J, Cariou A, Cronberg T, Moulaert VR, Deakin CD, Bottiger BW, Friberg H, Sunde K, Sandroni C. European Resuscitation Council and European Society of Intensive Care Medicine Guidelines for Post-resuscitation Care 2015: Section 5 of the European Resuscitation Council Guidelines for Resuscitation 2015. Resuscitation 2015; 95: 202-22.
22. Monsieurs KG, Nolan JP, Bossaert LL, Greif R, Maconochie IK, Nikolaou NI, Perkins GD, Soar J, Truhlář A, Wyllie J, Zideman DA. ERC Guidelines 2015 Writing GroupEuropean Resuscitation Council Guidelines for Resuscitation 2015: Section 1. Executive summary. Resuscitation 2015; 95: 1-80.
23. Voicu S, Sideris G, Deye N, Dillinger JG, Logeart D, Broche C, Vivien B, Brun PY, Capan DD, Manzo-Silberman S, Megarbane B, Baud FJ, Henry P. Role of cardiac troponin in the diagnosis of acute myocardial infarction in comatose patients resuscitated from out-of-hospital cardiac arrest. Resuscitation 2012; 83: 452-8.
24. Røsjø H, Vaahersalo J, Hagve T-A, Pettilä V, Kurola J, Omland T, and on behalf of the FINNRESUSCI Laboratory Study Group. Prognostic value of high-sensitivity troponin T levels in patients with ventricular arrhythmias and out-of-hospital cardiac arrest: data from the prospective FINNRESUSCI study. Crit Care 2014; 18: 605-614.
25. Oh SH, Kim YM, Kim HJ, Youn CS, Choi SP, Wee JH, Kim SH, Jeong WJ, Park KN.. Implication of cardiac marker elevation in patients who resuscitated from out-of-hospital cardiac arrest. Am J Emerg Med 2012; 30: 464–471.
26. Trinquart L, Ray P, Riou B, Teixeira A.. Natriuretic peptide testing in EDs for managing acute dyspnea: a meta-analysis. Am J Emerg Med 2011; 29: 757–767.
27. Rundgren M, Karlsson T, Nielsen N, Cronberg T, Johnsson P, Friberg H. Neuronspecifi c enolase and S-100B as predictors of outcome after cardiac arrest andinduced hypothermia. Resuscitation 2009; 80: 784–9.
28. Daubin C, Quentin C, Allouche S, Etard O, Gaillard C, Seguin A, Valette X, Parienti JJ, Prevost F, Ramakers M, Terzi N, Charbonneau P, du Cheyron D. Serum neuron-specifi c enolase as predictor of outcome in comatose cardiac-arrest survivors: a prospective cohort study. BMC Cardiovasc Disord 2011; 11: 48-60.
29. Rundgren M, Karlsson T, Nielsen N, Cronberg T, Johnsson P, Friberg H. Neuronspecifi c enolase and S-100B as predictors of outcome after cardiac arrest and induced hypothermia. Resuscitation 2009; 80: 784–9.
30. Lee BK, Jeung KW, Lee HY, Jung YH, Lee DH. Combining brain computedtomography and serum neuron specifi c enolase improves the prognostic per-formance compared to either alone in comatose cardiac arrest survivors treatedwith therapeutic hypothermia. Resuscitation 2013; 84: 1387–92.
31. Huntgeburth M, Adler C, Rosenkranz S, Zobel C, Haupt WF, Dohmen C, Reuter H. Changes in neuron-specifi c enolaseare more suitable than its absolute serum levels for the prediction of neurologicoutcome in hypothermia-treated patients with out-of-hospital cardiac arrest. Neurocrit Care 2014; 20: 358–66.
32. Stammet P, Devaux Y, Azuaje F, Werer C, Lorang C, Gilson G, Max M. Assessment of procalcitonin to predict outcome in hypothermia-treated patients after cardiac arrest. Crit Care Res Pract 2011; 2011: 631062.
33. Devaux Y, Stammet P, Friberg H, Hassager Ch, Kuiper MA, Wise MP, Nielsen N, and for the Biomarker subcommittee of the TTM trial (Target Temperature Management After Cardiac Arrest. MicroRNAs: new biomarkers and therapeutic targets after cardiac arrest? Crit Care 2015; 19: 54-61.
34. Niska R, Bhuiya F, Xu J. National Hospital Ambulatory Medical Care Survey: 2007 emergency department summary. Natl Health Stat Report 2010; 26:1-31.
35. Gandhi PU, Januzzi JL Jr. Can copeptin emerge from the growing shadow of the troponins? Eur Heart J 2015; 36: 333-6.
36. Swap CJ, Nagurney JT. Value and limitations of chest pain history in the evaluation of patients with suspected acute coronary syndromes. JAMA 2005; 294: 2623-9.
37. Thygesen K, Alpert JS, White HD. Universal defi nition of myocardial infarction. Eur Heart J 2007; 28: 2525–2538.
38. Thygesen K, Alpert JS, Jaffe AS, Simoons ML, Chaitman BR, White HD, Katus HA, Apple FS, Lindahl B, Morrow DA, Chaitman BA, Clemmensen PM, Johanson P, Hod H, Underwood R, Bax JJ, Bonow RO, Pinto F, Gibbons RJ, Fox KA, Atar D, Newby LK, Galvani M, Hamm CW, Uretsky BF, Steg PG, Wijns W, Bassand JP, Menasche P, Ravkilde J, Ohman EM, Antman EM, Wallentin LC, Armstrong PW, Januzzi JL, Nieminen MS, Gheorghiade M, Filippatos G, Luepker RV, Fortmann SP, Rosamond WD, Levy D, Wood D, Smith SC, Hu D, Lopez-Sendon JL, Robertson RM, Weaver D, Tendera M, Bove AA, Parkhomenko AN, Vasilieva EJ, Mendis S. Third universal defi nition of myocardial infarction. Eur Heart J 2012; 33: 2551–2567.
39. Steg PG, James SK, Atar D, Badano LP, Blömstrom-Lundqvist C, Borger MA, Di Mario C, Dickstein K, Ducrocq G, Fernandez-Aviles F, Gershlick AH, Giannuzzi P, Halvorsen S, Huber K, Juni P, Kastrati A, Knuuti J, Lenzen MJ, Mahaffey KW, Valgimigli M, van’t Hof A, Widimsky P, Zahger D. Task Force on the management of ST-segment elevation acute myocardial infarction of the European Society of Cardiology (ESC). ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation. Eur Heart J 2012; 33: 2569-619.
40. Kitamura M, Hata N, Takayama T, Hirayama A, Ogawa M, Yamashina A, Mera H, Yoshino H, Nakamura F, Seino Y. Different characteristics of cardiac biomarkers to decide and predict the culprit lesions in patients with suspicious acute coronary syndrome. Heart Vessels 2016; 31: 907-17.
41. Reichlin T, Twerenbold R, Reiter M, Steuer S, Bassetti S, Balmelli C, Winkler K, Kurz S, Stelzig C, Freese M, Drexler B, Haaf P, Zellweger C, Osswald S, Mueller C. Introduction of high-sensitivity troponin assays: impact on myocardial infarction incidence and prognosis. Am J Med 2012; 125: 1205–1213.
42. Leclercq F, Iemmi A, Kusters N, Lattuca B, Cayla G, Macia JC, Roubille F, Akodad M, Cristol JP, Dupuy AM. Copeptin and high-sensitivity cardiac troponin to exclude severe coronary stenosis in patients with chest pain and coronary artery disease. Am J Emerg Med 2016; 34: 493-8.
43. Lin S, Yokoyama H, Rac VE, Brooks SC. Novel biomarkers in diagnosing cardiac ischemia in the emergency department: a systematic review. Resuscitation 2012; 83: 684-91.
44. Tanaka T, Sohmiya K, Kitaura Y, Takeshita H, Morita H, Ohkaru Y, Asayama K, Kimura H. Clinical evaluation of point of care testing of heart-type fatty acid binding protein (H-FABP) for the diagnosis of acute myocardial infarction. J Immunoassay Immunochem 2006; 27: 225-238.
45. Pyati AK, Devaranavadagi BB, Sajjannar SL, Nikam SV, Shannawaz M, Sudharani. Heart-Type Fatty Acid Binding Protein: A Better Cardiac Biomarker than CK-MB and Myoglobin in the Early Diagnosis of Acute Myocardial Infarction. J Clin Diagn Res 2015; 9: BC08-11.
46. Bank IE, Dekker MS, Hoes AW, Zuithoff NP, Verheggen PW, de Vrey EA, Wildbergh TX, Timmers L, de Kleijn DP, Glatz JF, Mosterd A. Suspected acute coronary syndrome in the emergency room: Limited added value of heart type fatty acid binding protein point of care or ELISA tests: The FAME-ER (Fatty Acid binding protein in Myocardial infarction Evaluation in the Emergency Room) study. Eur Heart J Acute Cardiovasc Care 2016; 5: 364-74.
47. McCann CJ, Glover BM, Menown IB, Moore MJ, McEneny J, Owens CG, Smith B, Sharpe PC, Young IS, Adgey JA. Novel biomarkers in early diagnosis of acute myocardial infarction compared with cardiac troponin T. Eur Heart J 2008; 29: 2843–50.
48. Halade GV, Jin Y-F, Lindsey ML. Matrix Metalloproteinase (MMP)-9: a proximal biomarker for cardiac remodeling and a distal biomarker for infl ammation. Pharmacol Ther 2013; 139: 32–40.
49. Hagström E, James SK, Bertilsson M, Becker RC, Himmelmann A, Husted S, Katus HA, Steg PG, Storey RF, Siegbahn A, Wallentin L; PLATO Investigators. Growth differentiation factor-15 level predicts major bleeding and cardiovascular events in patients with acute coronary syndromes: results from the PLATO study. Eur Heart J 2016; 37: 1325-33.
50. Roffi M, Patrono C, Collet JP, Mueller C, Valgimigli M, Andreotti F, Bax JJ, Borger MA, Brotons C, Chew DP, Gencer B, Hasenfuss G, Kjeldsen K, Lancellotti P, Landmesser U, Mehilli J, Mukherjee D, Storey RF, Windecker S, Baumgartner H, Gaemperli O, Achenbach S, Agewall S, Badimon L, Baigent C, Bueno H, Bugiardini R, Carerj S, Casselman F, Cuisset T, Erol Ç, Fitzsimons D, Halle M, Hamm C, Hildick-Smith D, Huber K, Iliodromitis E, James S, Lewis BS, Lip GY, Piepoli MF, Richter D, Rosemann T, Sechtem U, Steg PG, Vrints C, Luis Zamorano J. Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology. 2015 ESC Guidelines for the management of acute coronary syndromes in patients presenting without persistent ST-segment elevation: Task Force for the Management of Acute Coronary Syndromes in Patients Presenting without Persistent ST-Segment Elevation of the European Society of Cardiology (ESC). Eur Heart J 2016; 37: 267-315.
51. Anderson JL, Adams CD, Antman EM, Bridges CR, Califf RM, Casey DE Jr, Chavey WE 2nd, Fesmire FM, Hochman JS, Levin TN, Lincoff AM, Peterson ED, Theroux P, Wenger NK, Wright RS, Jneid H, Ettinger SM, Ganiats TG, Philippides GJ, Jacobs AK, Halperin JL, Albert NM, Creager MA, DeMets D, Guyton RA, Kushner FG, Ohman EM, Stevenson W, Yancy CW. 2012 ACCF/AHA focused update incorporated into the ACCF/AHA 2007 guidelines for the management of patients with unstable angina/non-ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol 2013; 61: e179-347.
52. Reichlin T, Schindler C, Drexler B, Twerenbold R, Reiter M, Zellweger C, Moehring B, Ziller R, Hoeller R, Rubini Gimenez M, Haaf P, Potocki M, Wildi K, Balmelli C, Freese M, Stelzig C, Freidank H, Osswald S, Mueller C. One-hour ruleout and rule-in of acute myocardial infarction using high-sensitivity cardiac troponin T. Arch Intern Med 2012; 172: 1211–1218.
53. Mueller C. High-sensitivity cardiac troponin 2016: from a European perspective. Eur Heart J 2016; 37: 2388–2396.
54. Twerenbold R, Wildi K, Jaeger C, Gimenez MR, Reiter M, Reichlin T, Walukiewicz A, Gugala M, Krivoshei L, Marti N, Moreno Weidmann Z, Hillinger P, Puelacher C, Rentsch K, Honegger U, Schumacher C, Zurbriggen F, Freese M, Stelzig C, Campodarve I, Bassetti S, Osswald S, Mueller C. Optimal cutoff levels of more sensitive cardiac troponin assays for the early diagnosis of myocardial infarction in patients with renal dysfunction. Circulation 2015; 131: 2041–2050.
55. Thygesen K, Mair J, Mueller C, Huber K, Weber M, Plebani M, Hasin Y, Biasucci LM, Giannitsis E, Lindahl B, Koenig W, Tubaro M, Collinson P, Katus H, Galvani M, Venge P, Alpert JS, Hamm C, Jaffe AS. Recommendations for the use of natriuretic peptides in acute cardiac care: a position statement from the study group on biomarkers in cardiology of the ESC working group on acute cardiac care. Eur Heart J 2012; 33: 2001–2006.
56. Zeller T, Tunstall-Pedoe H, Saarela O, Ojeda F, Schnabel RB, Tuovinen T, Woodward M, Struthers A, Hughes M, Kee F, et al. High population prevalence of cardiac troponin I measured by a high-sensitivity assay and cardiovascular risk estimation: the MORGAM Biomarker Project Scottish Cohort. Eur Heart J 2014;35: 271-81.
57. Thygesen K, Alpert JS, Allan S, et al. Third universal defi nition of myocardial infarction. J Am Coll Cardiol 2012; 60: 1581–98.
58. Thygesen K, Mair J, Giannitsis E, Mueller C, Lindahl B, Blankenberg S, Huber K, Plebani M, Biasucci LM, Tubaro M, Collinson P, Venge P, Hasin Y, Galvani M, Koenig W, Hamm C, Alpert JS, Katus H, Jaffe AS; Study Group on Biomarkers in Cardiology of ESC Working Group on Acute Cardiac Care. How to use high-sensitivity cardiac troponins in acute cardiac care. Eur Heart J 2012; 33: 2252-7.
59. Suzuki T, Bossone E, Sawaki D, Jánosi RA, Erbel R, Eagle K, Nagai R. Biomarkers of aortic diseases. Am Heart J 2013; 165: 15-25.
60. Giannitsis E, Mair J, Christersson C, Siegbahn A, Huber K, Jaffe AS, Peacock WF, Plebani M, Thygesen K, Möckel M, Mueller C, Lindahl B; The Biomarker Study Group of the European Society of Cardiology (ESC) Acute Cardiovascular Care Association (ACCA). How to use D-dimer in acute cardiovascular care. Eur Heart J: Acute Cardiovascular Care 2017; 6: 69-80.
61. Kario K, Matsuo T and Kobayashi H. Which factors affect high D-dimer levels in the elderly? Thromb Res 1991; 62: 501–508.
62. Erbel R, Aboyans V, Boileau C, Bossone E, Bartolomeo RD, Eggebrecht H, Evangelista A, Falk V, Frank H, Gaemperli O, Grabenwöger M, Haverich A, Iung B, Manolis AJ, Meijboom F, Nienaber CA, Roffi M, Rousseau H, Sechtem U, Sirnes PA, Allmen RS, Vrints CJ; ESC Committee for Practice Guidelines. 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: Document covering acute and chronic aortic diseases of the thoracic and abdominal aorta of the adult. The Task Force for the Diagnosis and Treatment of Aortic Diseases of the European Society of Cardiology (ESC). Eur Heart J 2014; 35: 2873-926.
63. Sodeck G, Domanovits H, Schillinger M, et al. A D-dimer in ruling out acute aortic dissection: A systematic review and prospective cohort study. Eur Heart J 2007; 28: 3067–3075.
64. Eggebrecht H, Mehta RH, Metozounve H, Huptas S, Herold U, Jakob HG, Erbel R. Clinical implications of systemic infl ammatory response syndrome following thoracic aortic stent-graft placement. J Endovasc Ther 2008; 15: 135-43.
65. Suzuki T, Trimarchi S, Sawaki D, Grassi V, Costa E, Rampoldi V, Nagai R, Eagle K. Circulating transforming growth factor-beta levels in acute aortic dissection. J Am Coll Cardiol 2011; 58: 775.
66. Konstantinides S, Torbicki A, Agnelli G, Danchin N, Fitzmaurice D, Galie N, Gibbs JSR, Huisman M, Humbert M, Kucher N, Lang I, Lankeit M, Lekakis J, Maack Ch, Mayer E, Meneveau N, Perrier A, Pruszczyk P, Rasmussen LH, Schindler TH, Svitil P, Noordegraaf AV, Zamorano JL, Zompatori M. 2014 ESC Guidelines on the diagnosis and management of acute pulmonary embolism. The Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC). Endorsed by the European Respiratory Society (ERS). Eur Heart J 2014; 35: 3033-3073.
67. Meyer G, Vicaut E, DanaysT, Agnelli G, Becattini C, Beyer-Westendorf J, Bluhmki E, Bouvaist H, Brenner B, Couturaud F, Dellas C, Empen K, Franca A, Galiè N, Geibel A, Goldhaber SZ, Jimenez D, Kozak M, Kupatt C, Kucher N, Lang IM, Lankeit M, Meneveau N, Pacouret G, Palazzini M, Petris A, Pruszczyk P, Rugolotto M, Salvi A, Schellong S, Sebbane M, Sobkowicz B, Stefanovic BS, Thiele H, Torbicki A, Verschuren F, Konstantinides SV. Fibrinolysis for patients with intermediaterisk pulmonary embolism. N Engl J Med 2014; 370: 1402–1411.
68. Klok FA, Mos IC, Huisman MV. Brain-type natriuretic peptide levels in the prediction of adverse outcome in patients with pulmonary embolism: a systematic review and meta-analysis. Am J Respir Crit Care Med 2008; 178: 425–430.
69. Kucher N, Goldhaber SZ. Cardiac biomarkers for risk stratifi cation of patients with acute pulmonary embolism. Circulation 2003; 108: 2191–2194.
70. Lankeit M, Jiménez D, Kostrubiec M, Dellas C, Kuhnert K, Hasenfuss G, Pruszczyk P, Konstantinides S. Validation of N-terminal pro-brain natriuretic peptide cut-off values for risk stratifi cation of pulmonary embolism. Eur Respir J 2014; 43: 1669–1677.
71. Becattini C, Vedovati MC, Agnelli G. Prognostic value of troponins in acute pulmonary embolism: a meta-analysis. Circulation 2007; 116: 427–433.
72. Jiménez D, Uresandi F, Otero R, Lobo JL, Monreal M, Martı´ D, Zamora J, Muriel A, Aujesky D, Yusen RD. Troponin-based risk stratifi cation of patients with acute nonmassive pulmonary embolism: systematic review and metaanalysis. Chest 2009; 136: 974–982.
73. Lankeit M, Jiménez D, Kostrubiec M, Dellas C, Hasenfuss G, Pruszczyk P, Konstantinides S. Predictive value of the high-sensitivity troponin T assay and the simplifi ed pulmonary embolism severity index in hemodynamically stable patients with acute pulmonary embolism: a prospective validation study. Circulation 2011; 124: 2716–2724.
74. Agterof MJ, Schutgens RE, Snijder RJ, Epping G, Peltenburg HG, Posthuma EF, Hardeman JA, van der Griend R, Koster T, Prins MH, Biesma DH. Out of hospital treatment of acute pulmonary embolism in patients with a low NT-proBNP level. J Thromb Haemost 2010; 8: 1235–1241.
75. Lega JC, Lacasse Y, Lakhal L, Provencher S. Natriuretic peptides and troponins in pulmonary embolism: a meta-analysis. Thorax 2009; 64: 869-75.
76. Boscheri A, Wunderlich C, Langer M, Schoen S, Wiedemann B, Stolte D, Elmer G, Barthel P, Strasser RH. Correlation of heart-type fatty acid-binding protein with mortality and echocardiographic data in patients with pulmonary embolism at intermediate risk. Am Heart J 2010; 160: 294–300.
77. Dellas C, Puls M, Lankeit M, Schafer K, Cuny M, Berner M, Hasenfuss G, Konstantinides S. Elevated heart-type fatty acid-binding protein levels on admission predict an adverse outcome in normotensive patients with acute pulmonary embolism. J Am Coll Cardiol 2010; 55:2150–2157.
78. Lankeit M, Friesen D, Schafer K, Hasenfuss G, Konstantinides S, Dellas C. A simple score for rapid risk assessment of non-high-risk pulmonary embolism. Clin Res Cardiol 2013; 102: 73–80.
79. Dellas C, Tschepe M, Seeber V, Zwiener I, Kuhnert K, Schafer K, Hasenfuss G, Konstantinides S, Lankeit M. A novel H-FABP assay and a fast prognostic score for risk assessment of normotensive pulmonary embolism. Thromb Haemost 2014; 111: 996-1003.
80. Becattini C, Lignani A, Masotti L, Forte MB, Agnelli G. D-dimer for risk stratifi cation in patients with acute pulmonary embolism. J Thromb Thrombolysis 2012; 33: 48–57.
81. Crawford F, Andras A, Welch K, Sheares K, Keeling D, Chappell FM. D-dimer test for excluding the diagnosis of pulmonary embolism. Cochrane Database Syst Rev 2016; 8: CD010864.
82. Lankeit M, Kempf T, Dellas C, Cuny M, Tapken H, Peter T, Olschewski M, Konstantinides S,Wollert KC. Growth differentiation factor-15 for prognostic assessment of patients with acute pulmonary embolism. Am J Respir Crit Care Med 2008; 177: 1018–1025.
83. Kostrubiec M, Łabyk A, Pedowska-Włoszek J, Pacho S, Wojciechowski A, Jankowski K, Ciurzyński M, Pruszczyk P. Assessment of renal dysfunction improves troponin-based short-term prognosis in patients with acute symptomatic pulmonary embolism. J Thromb Haemost 2010; 8: 651–658.
84. Kostrubiec M, Łabyk A, Pedowska-Włloszek J, Dzikowska-Diduch O, Wojciechowski A, Garlińska M, Ciurzyński M, Pruszczyk P. Neutrophil gelatinase-associated lipocalin, cystatin C and eGFR indicate acute kidney injury and predict prognosis of patients with acute pulmonary embolism. Heart 2012; 98: 1221–1228.
85. Galiè N, Humbert M, Vachiery JL, Gibbs S, Lang I, Torbicki A, Simonneau G, Peacock A, Vonk Noordegraaf A, Beghetti M, Ghofrani A, Gomez Sanchez MA, Hansmann G, Klepetko W, Lancellotti P, Matucci M, McDonagh T, Pierard LA, Trindade PT, Zompatori M, Hoeper M, Aboyans V, Vaz Carneiro A, Achenbach S, Agewall S, Allanore Y, Asteggiano R, Paolo Badano L, Albert Barberà J, Bouvaist H, Bueno H, Byrne RA, Carerj S, Castro G, Erol Ç, Falk V, Funck-Brentano C, Gorenfl o M, Granton J, Iung B, Kiely DG, Kirchhof P, Kjellstrom B, Landmesser U, Lekakis J, Lionis C, Lip GY, Orfanos SE, Park MH, Piepoli MF, Ponikowski P, Revel MP, Rigau D, Rosenkranz S, Völler H, Luis Zamorano J. 2015 ESC/ERS Guidelines for the diagnosis and treatment of pulmonary hypertension: The Joint Task Force for the Diagnosis and Treatment of Pulmonary Hypertension of the European Society of Cardiology (ESC) and the European Respiratory Society (ERS): Endorsed by: Association for European Paediatric and Congenital Cardiology (AEPC), International Society for Heart and Lung Transplantation (ISHLT). Eur Heart J 2016; 37: 67-119.
86. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JG, Coats AJ, Falk V, González-Juanatey JR, Harjola VP, Jankowska EA, Jessup M, Linde C, Nihoyannopoulos P, Parissis JT, Pieske B, Riley JP, Rosano GM, Ruilope LM, Ruschitzka F, Rutten FH, van der Meer P. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J 2016; 37: 2129-200.
87. Kelder JC, Cramer MJ, Verweij WM, Grobbee DE, Hoes AW. Clinical utility of three B-type natriuretic peptide assays for the initial diagnostic assessment of new slow-onset heart failure. J Card Fail 2011; 17: 729–734.
88. Stokes NR, Dietz BW, Liang JJ. Cardiopulmonary laboratory biomarkers in the evaluation of acute dyspnea. Open Access Emerg Med 2016; 8: 35-45.
89. Maisel A, Mueller C, Nowak R, Peacock WF, Landsberg JW, Ponikowski P, Mockel M, Hogan C, Wu AH, Richards M, Clopton P, Filippatos GS, Di Somma S, Anand I, Ng L, Daniels LB, Neath SX, Christenson R, Potocki M, McCord J, Terracciano G, Kremastinos D, Hartmann O, von Haehling S, Bergmann A,Morgenthaler NG, Anker SD. Mid-region pro-hormone markers for diagnosis and prognosis in acute dyspnea: results from the BACH (Biomarkers in Acute Heart Failure) trial. J Am Coll Cardiol 2010; 55: 2062–2076.
90. Arrigo M, Gayat E, Parenica J, Ishihara S, Zhang J, Choi D-J, Park JJ, Alhabib KF, Sato N, Miro O, Maggioni AP, Zhang Y, Spinar J, Cohen-Solal A, Iwashyna TJ, Mebazaa A. and on behalf of the GREAT Network. Precipitating factors and 90-day outcome of acute heart failure: a report from the intercontinental GREAT registry. Eur J Heart Fail 2017; 19: 201–208.
91. La Vecchia L, Mezzena G, Zanolla L, Paccanaro M, Varotto L, Bonanno C, Ometto R. Cardiac troponin I as diagnostic and prognostic marker in severe heart failure. J Heart Lung Transplant 2000;19:644–652.
92. Diercks DB, Peacock WF, Kirk JD, Weber JE. ED patients with heart failure: identifi cation of an observational unit-appropriate cohort. Am J Emerg Med 2006; 24: 319-24.
93. Globits S, Frank H, Pacher B, Huelsmann M, Ogris E, Pacher R. Atrial natriuretic peptide release is more dependent on atrial fi lling volume than on fi lling pressure in chronic congestive heart failure. Am Heart J 1998; 135: 592–597.
94. de Boer RA, Yu L, van Veldhuisen DJ. Galectin-3 in cardiac remodeling and heart failure. Curr Heart Fail Rep 2010; 7: 1–8.
95. van Kimmenade RR, Januzzi JL Jr, Ellinor PT, Sharma UC, Bakker JA, Low AF, Martinez A, Crijns HJ, MacRae CA, Menheere PP, Pinto YM. Utility of amino-terminal pro-brain natriuretic peptide, galectin-3, and apelin for the evaluation of patients with acute heart failure. J Am Coll Cardiol 2006; 48: 1217-24.
96. Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE Jr, Drazner MH, Fonarow GC, Geraci SA, Horwich T, Januzzi JL, Johnson MR, Kasper EK, Levy WC, Masoudi FA, McBride PE, McMurray JJ, Mitchell JE, Peterson PN, Riegel B, Sam F, Stevenson LW, Tang WH, Tsai EJ, Wilkoff BL. 2013 ACCF/AHA guideline for the management of heart failure: executive summary: a report of the American College of Cardiology Foundation/American Heart Association Task Force on practice guidelines. Circulation 2013; 128: 1810-52.
97. Magnani G, O’Donoghue M, Brunwald E, Steen D, Jarolim P, Lukas MA, White H, Lewis B, de Winter R, Zhou J, Im K, Cannon Ch, Morrow D. Galectin-3 for heart failure risk stratifi cation in patients after acute coronary syndromes: insights from the Solid-TIMI 52 trial. J Am Coll Cardiol 2015; 65(10 Suppl): A773.
98. Benoit JL, Hicks CW, Engineer RS, Hart KW, Lindsell CJ, Peacock WF. ST2 in emergency department patients with noncardiac dyspnea. Acad Emerg Med 2013; 20: 1207–1210.
99. Dieplinger B, Egger M, Haltmayer M, Kleber ME, Scharnagl H, Silbernagel G, de Boer RA, Maerz W, Mueller T. Increased soluble ST2 predicts long-term mortality in patients with stable coronary artery disease: results from the Ludwigshafen risk and cardiovascular health study. Clin Chem 2014; 60: 530–540.
100. Maisel A, Neath SX, Landsberg J, Mueller C, Nowak RM, Peacock WF, Ponikowski P, Mőckel M, Hogan C, Wu AHB, Richards M, Clopton P, Filippatos GS, Di Somma S, Anand I, Ng LL, Daniels LB, Christenson RH, Potocki M, McCord J, Terracciano G, Hartmann O, Bergmann A, Morgenthaler NG, Anker SD. Use of procalcitonin for the diagnosis of pneumonia in patients presenting with a chief complaint of dyspnoea: results from the BACH (Biomarkers in Acute Heart Failure) trial. Eur J Heart Fail 2012; 14: 278–286.
101. Wollert KC. Tailored therapy for heart failure: the role of biomarkers. Eur Heart J 2012; 33: 2246-8.
102. Demissei BG, Valente MAE, Cleland JG, O’Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Davison B, Givertz MM, Bloomfi eld DM, Dittrich H, van der Meer P, van Veldhuisen DJ, Hillege HL, Voors AA. Optimizing clinical use of biomarkers in high-risk acute heart failure patients. Eur J Heart Fail 2016; 18: 269–280.
103. Demissei BG, Postmus D, Cleland JG, O’Connor CM, Metra M, Ponikowski P, Teerlink JR, Cotter G, Davison BA, Givertz MM, Bloomfield DM,van Veldhuisen DJ, Dittrich HC, Hillege HL, Voors AA. Plasma biomarkers to predict or rule out early post-discharge events after hospitalization for acute heart failure. Eur J Heart Fail 2017; 19: 728- 738.
104. Chioncel O, Collins SP, Greene SJ, Ambrosy AP, Vaduganathan M, Macarie C, Butler J, Gheorghiade M. Natriuretic peptide-guided management in heart failure. J Cardiovasc Med 2016;17: 556-68.
105. van Kimmenade RRJ, Januzzi Jr JL. Emerging Biomarkers in Heart Failure. Clinical Chemistry 2012; 58: 127-138.
106. Goldhaber SZ. Fine-tuning risk stratifi cation for acute pulmonary embolism with cardiac biomarkers. J Am Coll Cardiol 2010; 55: 2158-9.
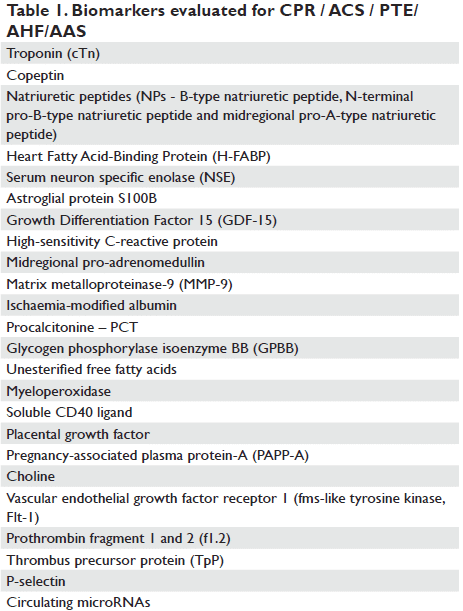
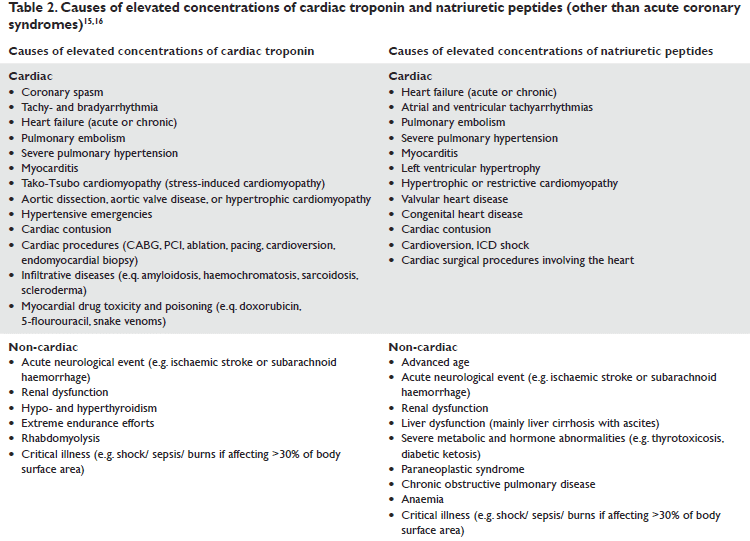
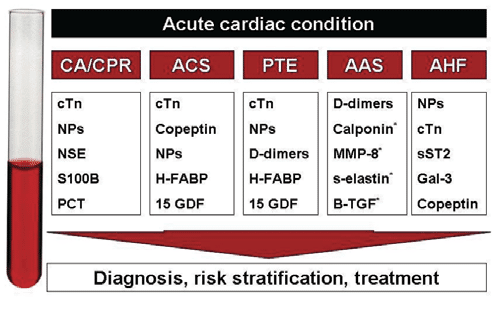
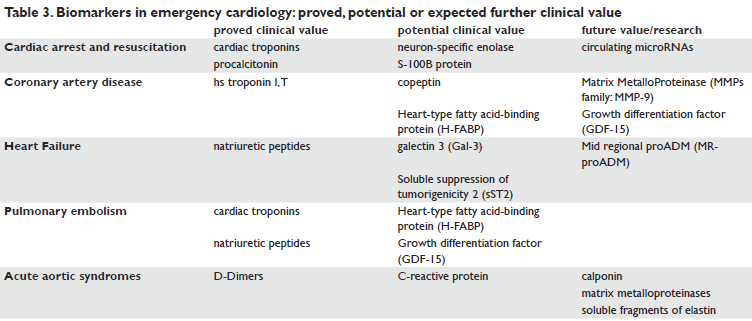
 This work is licensed under a
This work is licensed under a