Mihaela Octavia Popa1, Corneliu Iorgulescu2
1 “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania
2 Department of Cardiology, Clinical Emergency Hospital, Bucharest, Romania
Abstract: Barlow’s disease is echocardiographically characterized by thickened and prolapsed mitral leaflets due to di-ffuse excess of tissue caused by myxomatous degeneration. We report the case of a 65-year-old male with lumbar pain as presenting feature of Barlow’s disease. The patient was admitted for dyspnoea at rest and reported acute lumbar pain before admission. Clinical examination and EKG changes were consistent with atrial fibrillation with rapid ventricular response. The abdominal-pelvic CT scan showed suggestive aspect of splenic infarction. After admission, the patient suffered an episode of transitory aphasia compatible with a transient ischaemic attack. Echocardiography revealed prolapse of both mitral leaflets, moderate-severe mitral regurgitation, LVEF of 40% and left atrial enlargement. The transesophageal echocardiography con-firmed Barlow’s disease and asserted there were no thrombi in the left appendage. During hospitalization and after discharge the patient received anticoagulation therapy (high molecular weight heparin followed by oral anticoagulant), beta-blocker and angiotensin-converting-enzyme inhibitor. Cardioversion was performed after one month and the patient remained in sinus rhythm during two years follow-up. After cardioversion the LVEF and LV dimensions normalised. The therapeutic approach is mitral valve repair which was temporized due to reversed systolic dysfunction and NYHA class I symptoms. The particularity of the case consists in the presentation with lumbar pain and the reversibility of LV dysfunction to sinus rhythm restoration, proving tachycardiomyopathy.
Keywords: Barlow’s disease, atrial fibrillation, mitral regurgitation, splenic infarction, heart failure, transient ischaemic attack.
BACKGROUND
Degenerative mitral valve regurgitation or Barlow’s disease is characterized by diffuse excess tissue cau-sed by myxomatous degeneration1,2. The mitral valve leaflets are thickened, while annular dilatation with varying degrees of annular calcifi cation and lengthening of the chordae tendineae may be observed. The valve is generally enlarged (especially the posterior leafl et, which can reach the same dimensions as the opposite leafl et) and multiple segments are usually affected3,4. These changes generate mitral valve prolapse in the left atrium beyond the mitral annulus with leaflet mal-coaptation which can result in varying degrees of mi-tral regurgitation5,7. It occurs in approximately 2-3% of the population and is equally distributed between men and women, irrespective of race8.
CASE PRESENTATION
M.I., a 65-year-old male, was admitted to hospital for one week history of dyspnoea at minimal effort and at rest. The patient reported intense left lumbar pain accompanied by diaphoresis occurring three weeks before admission. The diagnosis at that time was renal colic – and he received NSAIDs and antispastics. He also reported a transient episode of intermittent clau-dication with the duration of 2-3 hours.
Clinical exam revealed slightly diminished murmur and pulmonary rales bilaterally in the bases, rapid irre-gular heart sounds with a heart rate of 150/minute and mid-systolic click followed by end-systolic murmur at the apex, while peripheral pulses were palpable bilate-rally. Blood pressure (BP) at arrival was 140 over 75 mmHg. An examination of his renal system was unre-markable with bilaterally absent Giordano maneuver.
The EKG changes were consistent with atrial fi-brillation with rapid ventricular rate of response (Figu-re 1). Standard blood test results were within normal limits, except for mild leukocytosis. Urine tests were normal and the urine cultures came negative.
Echocardiography – performed after obtaining heart rate control – showed prolapse of both mitral valve leaflets with moderate-severe mitral regurgitati-on (eccentric jet reaching the posterior wall of the left atrium), severe left atrial enlargement (46/59/50 mm), left atrium volume of 91 ml/sqm and atrial fibrillation. There was also ultrasound evidence of moderate systolic dysfunction (left ventricular ejection fraction (LVEF) of 40%, left ventricular end-diastolic volume (LVEDV) of 160 ml, left ventricular end-systolic volu-me (LVESV) of 93 ml and left ventricular end-systolic diameter (LVESD) of 55 mm) due to left ventricle (LV) diffuse kinetics modifications (Figure 2 and Figure 3).
An abdominal-pelvic CT scan found a hypodense region (49/21mm) in the inferior splenic pole suggestive of infarction and minor bilateral pleural effusion. There were no calculi in the urinary tract (Figure 4).
The patient was started on anticoagulation therapy with i.v. sodic heparin followed by oral anticoagulants to maintain international normalized ratio (INR) 2-3. He also received metoprolol succinate titrated as to achieve a heart rate at rest of about 80 bpm and rami-pril as basic treatment for his heart failure.
Under treatment with oral anticoagulants, at an INR of 2.3, he suffered an episode of aphasia. The neurologic exam diagnosed a left Sylvian stroke and recommended urgent CT that asserted the absence of intracerebral hemorrhage (Figure 5). The aphasia resolved spontaneously and the patient was continued on heparin and then switched to oral anticoagulants at an INR between 3 and 4.
The transesophageal echocardiography (TEE) show-ed a suggestive aspect for Barlow’s disease: thickening of the leaflets with diffuse tissue excess, multi-seg-ment, generalised prolapse of different grades of all scallops of both mitral leaflets, exceeding the mitral annulus plan in systole (Figure 6). The left atrium was severely dilated with moderate spontaneous contrast, but without thrombi in the left appendage– after one week of sodic heparin therapy (Figure 7). The mitral regurgitation was considered moderate-severe – PISA radius of 8.3 mm, effective regurgitant orifice area (EROA) of 0.23 cm2, regurgitant volume (RV) of 31 ml (probably underestimated due to the eccentric re-gurgitation) (Figure 8, Figure 9). Doppler ultrasound revealed normal carotid and femoral arteries.
The final diagnosis is Barlow’s disease with mode-rate-severe mitral regurgitation complicated with mild left ventricular systolic dysfunction and left atrial di-latation, atrial fibrillation, splenic and cerebral embo-lisms.
During the hospitalization, under treatment with anticoagulation therapy (high molecular weight hepa-rin followed by oral anticoagulant), beta blocker and angiotensin converting enzyme inhibitor the patient had a favorable evolution: eupnoea, heart rate control (90 bpm) and BP of 100/60 mmHg, effi cient anticoa-gulation and complete remission of the neurological symptoms. The patient was discharged with NYHA class I symptoms.
After discharge, he received ramipril 5 mg od, me-toprolol succinate 100 mg od and acenocumarol to maintain an of INR 3-4 in order to achieve a better prevention for embolisms, since the transient ischae-mic attack (TIA) occurred at an INR of 2.3. After one month of oral anticoagulation, a new TEE was perfor-med to ensure the safety of the cardioversion. The in-vestigation confirmed lack of thrombi in the left atrium and appendage and cardioversion was performed successfully. No antiarrhythmic was added to metopro-lol since it was the first documented atrial fibrillation episode in this patient. INR was kept to 3-4 another month after cardioversion and then it was lowered to 2-3 indefi nitely. LVEF normalized at one month after cardioversion (Figure 10), while the quantitative parameters for mitral regurgitation modifi ed – PISA radius of 10 mm, EROA 0.3 of cm2, RV of 56 ml (Figure 11). During two years follow-up (clinical exam, echocardi-ography and ECG Holter every six months), the pati-ent remained in sinus rhythm and presented one epi-sode of paroxysmal atrial fibrillation in extreme stress conditions (a burglar trying to enter his house).
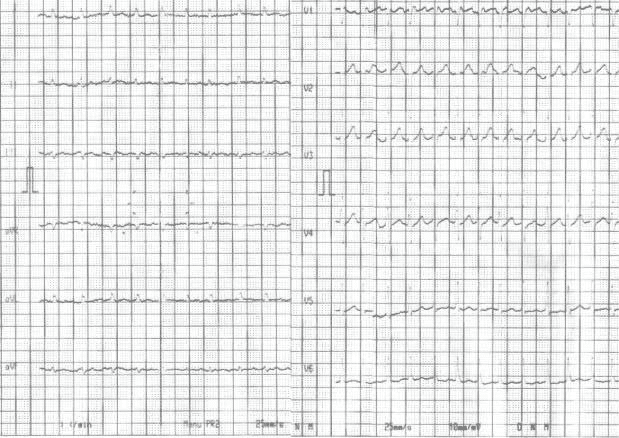
Figure 1. Electrocardiogram – atrial fibrillation with rapid rate of ventricu-lar response; heart rate of 150/minute.
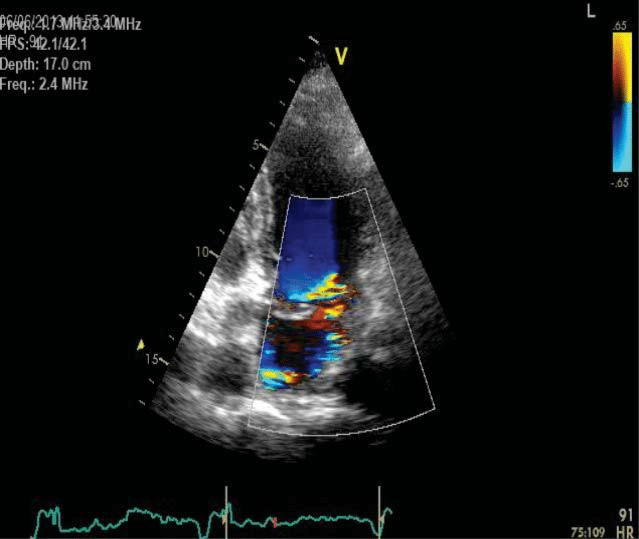
Figure 2. Transthoracic echocardiography apical four chamber view – moderate severe mitral regurgitation, severe left atrial enlargement.
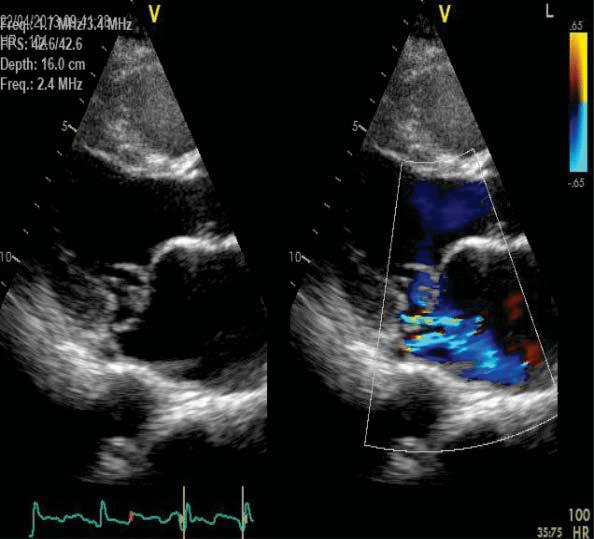
Figure 3. Transthoracic echocardiography parasternal long axis view – myxomatous degeneration and prolapse of both anterior and posterior mitral leaflets, moderate severe mitral regurgitation.
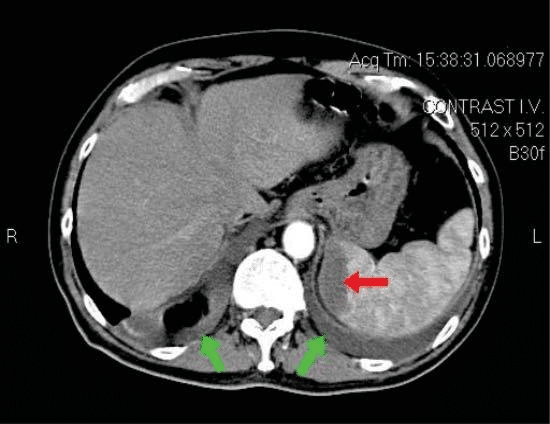
Figure 4. Abdominal pelvic CT scan – red arrow: hypodense region – splenic infarction, green arrows: minor bilateral pleural effusion.
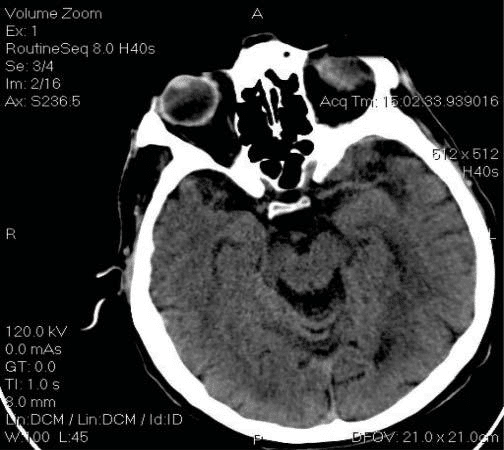
Figure 5. Cerebral CT scan – absence of intracerebral hemorrhage.
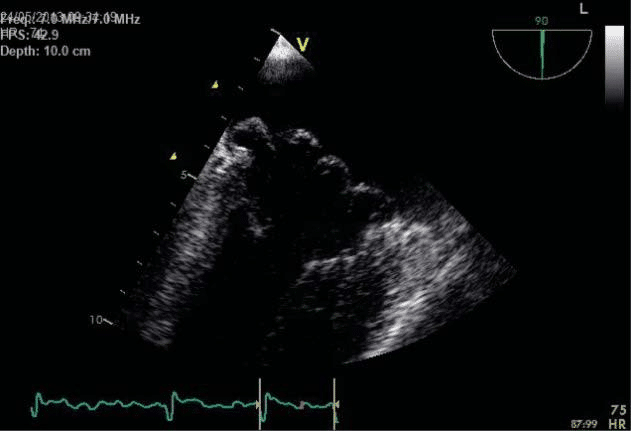
Figure 6. Transesophageal echocardiography, bicommisural view –multi-segmentar prolapse of mitral valve.
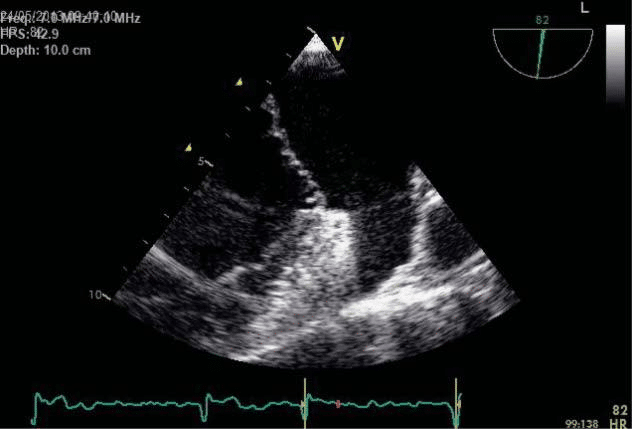
Figure 7. Transesophageal echocardiography focusing on the left append-age: no spontaneous contrast or thrombi.
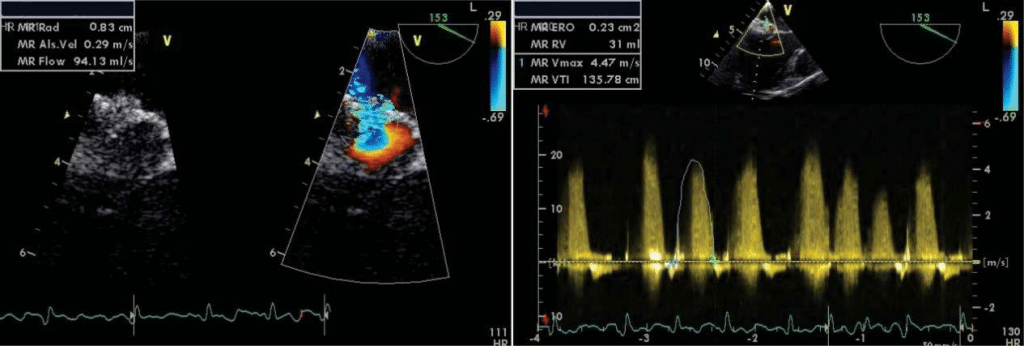
Figure 8. Transesophageal echocardiography for measuring quantitative parameters for MR – 8 A and 8 B moderate severe mitral regurgitation PISA radius=8.3 mm, EROA 0.23 cm2, RV 31 ml.
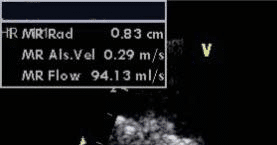
Figure 9. Transesophageal echocardiography, long axis view with colour Doppler – important mitral regurgitation with large eccentric jet and Coan-da effect.
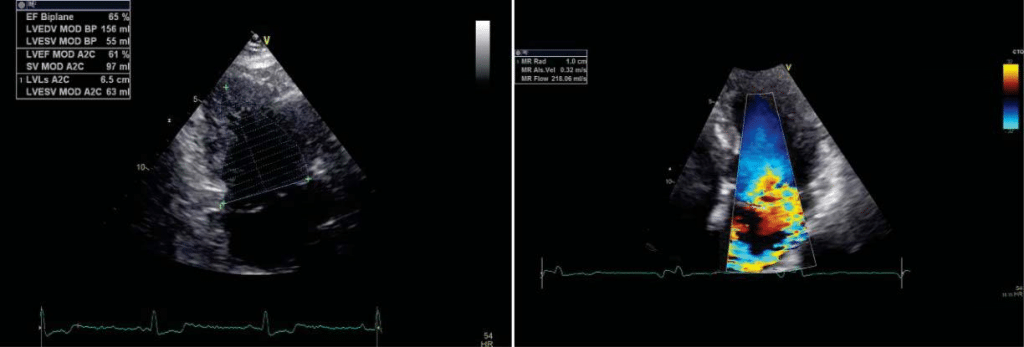
Figure 10. Transthoracic echocardiography – improved systolic function LVEF=65%, LVEDV 156 ml, LVESV 55 ml, LVESD=37 mm.
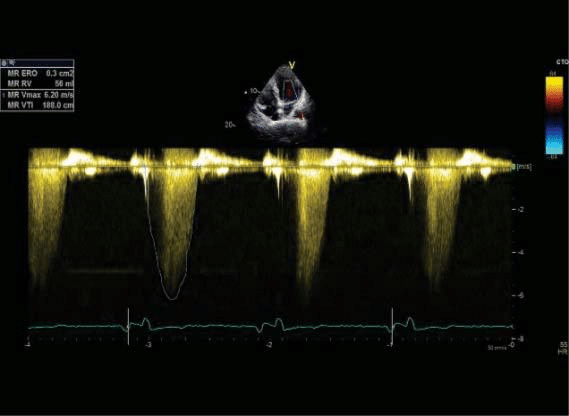
Figure 11. Transthoracic echocardiography apical four chamber view for measuring quantitative parameters for mitral regurgitation – 11 A and 11 B moderate severe mitral regurgitation PISA radius=10 mm, EROA 0.3 cm2, RV 56 ml.
DISCUSSION
For this patient, the presenting feature was lumbar pain which proved to be consecutive to a splenic infarction. In addition, he had complained of a transiently occur-ring intermittent claudication. No clinical or echo Doppler signs of peripheral artery disease were found at admission and both manifestations were considered to be of cardio-embolic origin, taking into considerati-on the patient’s heart condition (mitral valve prolapse and atrial fi brillation) as well as the normal aorta and peripheral arteries. Another particularity of the case was the TIA occurring under correct anticoagulation during the patient’s admission. Whether this was ca-used by the embolism from the faulty surfaced mitral valve leaflets9,10 or by thromboembolism from the left atrium has been debatable. In the end, we consider the TIA to be thromboembolic, even if the TEE showed absence of thrombi, since the TEE was performed af-ter the TIA had occurred. In our opinion the presence of rapidly occurring multiple embolisms and the ex-cellent response on the long time under anticoagulant therapy makes embolism with valve material unlikely.
Medical treatment indications are for symptoma-tic patients to relieve symptomatology and prevent complications. They include beta-blockers for rate control and oral anticoagulation with INR value at 2-3 for recurrent embolisms. Surgical therapy targets ex-clusively patients with mitral valve prolapse and mi-tral regurgitation and follows the European Society of Cardiology guidelines (2012) – symptomatic patients with a LVEF over 30% and LVESD under 55 mm and asymptomatic patients with LV dysfunction (LVESD ≥45 mm and/or LVEF ≤60%) have a class I level of in-dication for mitral repair11. This technique is preferred to mitral valve replacement because the native valve is kept and the patient is spared of the risks of chronic anticoagulation11-14. In this case, although initially our patient respected the criteria for mitral valve repair recommendation – symptomatic heart failure, LVEF of 40% and LVESD of 55 mm, due to the recent TIA we decided to initiate conservative treatment with beta blockers and angiotensin converting enzyme inhibitors for rate control and heart failure, anticoagulation with sodic heparin followed by oral anticoagulants for em-bolism prevention, while a cardioversion was planned at one month. The patient’s condition improved under treatment and the he was discharged with NYHA class I symptomatology. After cardioversion, the patient’s LVEF and LV dimensions normalized at one month. This feature of LVEF normalization after cardioversi-on – and not after rate control – indicates a compo-nent of arrhythmia-induced cardiomyopathy, a poten-tially reversible condition after the treatment of the arrhythmia15,16. The patient’s surgical indication had changed, as the LV volumes and function became normal. Theoretically, with atrial fi brillation mitral valve surgical repair is a Class IIa indication. After discussing this option with the patient, we decided to continue conservatively with a close follow-up. The patient was NYHA I, with normal LV volumes and function and he remained in sinus rhythm during two year follow-up.
CONCLUSIONS
The first symptoms, described as a renal colic and in-termittent claudication were misleading. The CT scan revealed a splenic infarction, and echo Doppler for peripheral arteries was normal. In correlation with the patient’s heart condition, it was suggestive for car-dio-embolisms. A correct diagnosis algorithm led to the fi nal conclusion of Barlow’s disease with moderate severe mitral regurgitation, mild LV dysfunction, atrial fibrillation and multiple systemic embolisms (splenic, cerebral and lower limbs). The therapeutic approach of the disease is mitral valve repair which was temporized due to reversed systolic dysfunction and NYHA class I symptoms. The particularity of the case consists in the atypical clinical presentation and the presence of tachycardiomyopathy as the cause of LV dysfunction in a case of Barlow disease with moderate – severe mitral regurgitation.
Conflict of interest: none declared.
References
1. Anyanwu AC, Adams DH. Etiologic classification of degenerative mi-tral valve disease: Barlow’s disease and fibroelastic deficiency. Semin Thorac Cardiovasc Surg 2007;19:90–96
2. Barlow JB, Pocock WA. Billowing, floppy, prolapsed or flail mitral valves? Am J Cardiol 1985; 55:501–502
3. Barlow JB, Bosman CK. Aneurysmal protrusion of the posterior leafl et of the mitral valve. An auscultatory-electrocardiographic syn-drome. Am Heart J 1966; 71(2):166–178
4. Carpentier AF, Pellerin M, Fuzellier JF, Relland JY. Extensive calcifica-tion of the mitral valve anulus: pathology and surgical management. J Thorac Cardiovasc Surg 1996; 111:718–729. discussion 729–730
5. Enriquez-Sarano M, Akins CW, Vahanian A. Mitral regurgita-tion. Lancet 2009; 373:1382–1394.
6. Anders S, Said S, Schulz F, Puschel K. Mitral valve prolapse syndrome as cause of sudden death in young adults. Forensic Sci Int 2007; 171:127–130
7. Schoen FJ. Evolving concepts of cardiac valve dynamics: the contin-uum of development, functional structure, pathobiology, and tissue engineering. Circulation 2008; 118:1864–1880
8. Flack JM, Kvasnicka JH, Gardin JM, Gidding SS, Manolio TA, Jacobs DR Jr. Anthropometric and physiologic correlates of mitral valve prolapse in a biethnic cohort of young adults: the CARDIA study. Am Heart J 1999; 138:486
9. Boudoulas KD, Boudoulas H. Floppy mitral valve (FMV)/mitral valve prolapse (MVP) and the FMV/MVP syndrome: pathophysio-logic mechanisms and pathogenesis of symptoms. Cardiology 2013; 126(2):69-80
10. Boudoulas KD, Pitsis AA, Boudoulas H. Floppy Mitral Valve (FMV) – Mitral Valve Prolapse (MVP) – Mitral Valvular Regurgitation and FMV/ MVP Syndrome. Hellenic J Cardiol 2016; 57(2):73-85
11. Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schafers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Win-decker S, Zamorano JL, Zembala M; The Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thorac-ic Surgery (EACTS). Guidelines on the management of valvular heart disease. Eur Heart J 2012; 33, 2469–2473
12. David TE, Ivanov J, Armstrong S, Rakowski H. Late outcomes of mitral valve repair for floppy valves: Implications for asymptomatic patients. J Thorac Cardiovasc Surg 2003; 125:1143–1152
13. Enriquez-Sarano M, Schaff HV, Orszulak TA, Tajik AJ, Bailey KR, Frye RL. Valve repair improves the outcome of surgery for mitral re-gurgitation. A multivariate analysis. Circulation 1995; 91:1022–1028
14. Gillinov AM, Blackstone EH, Nowicki ER, Slisatkorn W, Al-Dossari G, Johnston DR, George KM, Houghtaling PL, Griffi n B, Sabik JF 3rd, Svensson LG. Valve repair versus valve replacement for degenerative mitral valve disease. J Thorac Cardiovasc Surg 2008; 135:885–893
15. Walker NL, Cobbe SM, Birnie DH. Tachycardiomyopathy: a diagno-sis not to be missed. Heart 2004; 90:e7
16. Gopinathannair R, Etheridge SP, Marchlinski FE, Spinale FG, Lak-kireddy D, Olshansky B. Arrhythmia-Induced Cardiomyopathies: Mechanisms, Recognition, and Management. J Am Coll Cardiol 2015; 66(15): 1714–1728.
 This work is licensed under a
This work is licensed under a