Nicoleta Oprescu1, Miruna M. Micheu1, Alina I. Scarlatescu1, Gabriela Nicula1, Maria Dorobantu1
1 Clinical Emergency Hospital, Bucharest, Romania
Abstract: Objective – Myocardial perfusion can be safely assessed using contrast echocardiography. Our aim is to demonstrate the diagnostic value of resting myocardial contrast echocardiography (MCE), and its correlation with scintigraphy, transthoracic echocardiography and coronary angiography. Method – For the purpose of this study (case-control) we selected two patients, one control patient having cardiovascular risk factors but no ischemic heart disease and another patient with prior myocardial infarction. Left ventricle microperfusion was quantified using multiple techniques: echocardiography, myocardial contrast echocardiography (MCE) and myocardial scintigraphy with 99mTc- tetrofosmin radiofarmaceutic. Both patients underwent coronary angiography immediately after hospital admission. As a contrast agent for MCE we used sulphur hexafluoride microbubbles (Sonovue®); myocardial perfusion was assessed using quantitative perfusion parameters (peak intensity and time to peak) and further analyzed by dedicated software. Results – We obtained lower peak intensity and longer time to peak values in case of the ischemic patient compared to control, thus demonstrating myocardial microcirculation dysfunction. Results obtained by MCE are concordant with the results obtained by standard echocardiography, coronary angiography and myocardial scintigraphy regarding perfusion, regional motility and systolic wall thickening. Conclusions – MCE brings useful information regarding myocardial microcirculation.
Keywords: microperfusion, echocardiography, myocardial scintigraphy, acute coronary syndrome.
INTRODUCTION
Cardiovascular disease is the leading global cause of death, ischemic heart disease (IHD) having a major impact on morbidity and mortality. Atherosclerosis is one of the main determinants of IHD, affecting both major coronary arteries and coronary microcirculation. Myocardial contrast echocardiography (MCE) is a noninvasive technique that uses microbubbles (approximately 1-8 μm in diameter) which remain in the systemic circulation for ~3-5 min after venous injection, having intravascular rheological features similar to those of erythrocytes. They are circulating in the intravascular space, causing myocardial opacifi cation which is used for the evaluation of myocardial perfusion1. Any change in signal intensity reflects a change in myocardial blood flow2. After destruction of the contrast with a fl ash, the signal intensity is anticipated to return to normal after 5-7 cardiac cycles3. The rate of microbubble replenishment can be assessed qualitatively, but also quantitatively by analyzing the change in signal intensity in a period of time for a specific region of interest (ROI), using a logarithmic curve analysis. Myocardial blood flow is considered the product of plateau signal intensity and rate of replenishment. Previous studies demonstrated that a slower rate of replenishment and a lower plateau signal intensity is characteristic for impaired myocardial blood flow4.
CASE REPORT
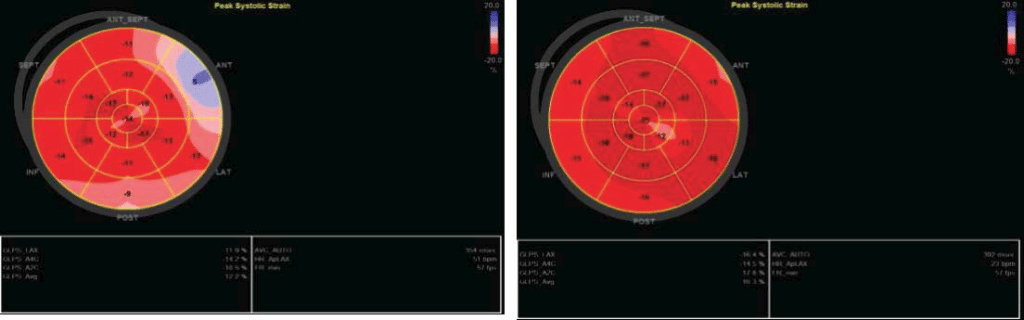
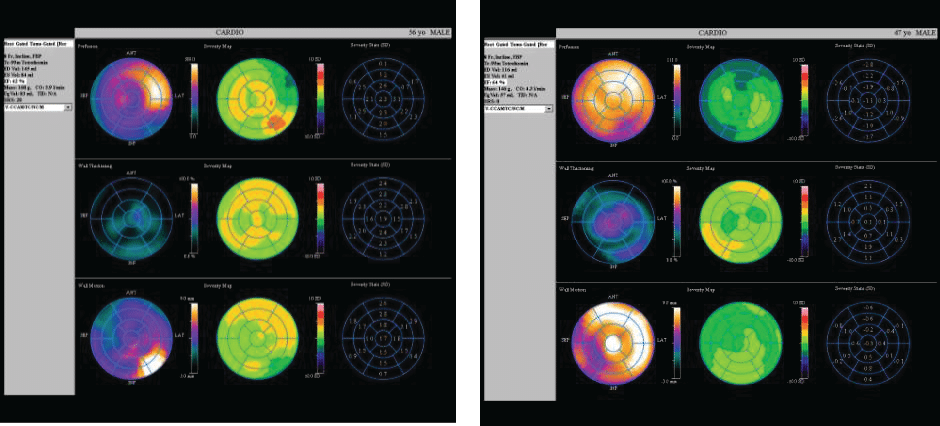
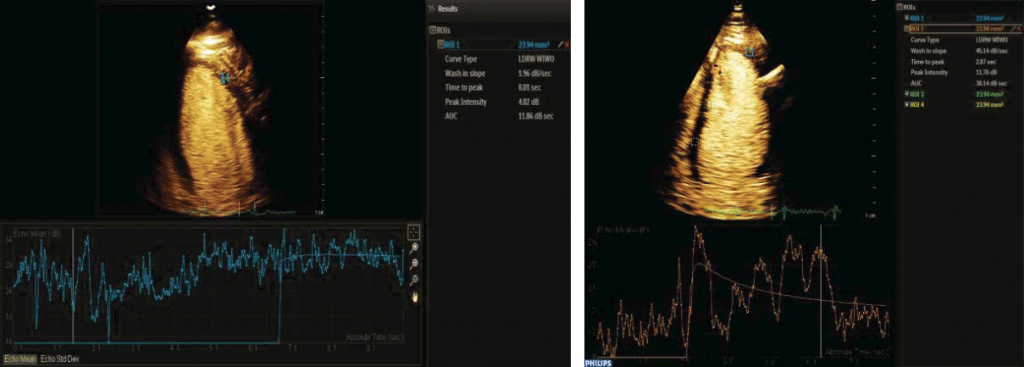
In this case control study we evaluated two male patients, in the 5th decade of life, same constitution (BMI= 30 kg/m2), smokers, both dyslipidemic but without diabetes. One patient (the IHD patient – case) was hypertensive, with acute anterior myocardial infarction three months before when was treated by primary angioplasty (3 hours after pain onset) and drug-eluting stent placement for 90% stenosis of left anterior descending artery in the second segment. Transthoracic echocardiography revealed severe hypokinesia of the anterior wall and mild left ventricular systolic dysfunction, EF=40%. The second patient (control) was admitted to the emergency department at the same time as the first, with suspicion of acute coronary syndrome. He presented with anterior chest pain, a mild ST elevation on ECG, but negative cardiac biomarkers and no wall motion abnormalities on transthoracic echocardiography; coronary angiography at admission showed coronary arteries without significant stenosis. Both patients were reevaluated 3 months after the primary event using transthoracic echocardiography, global longitudinal strain, MCE, and myocardial scintigraphy with 99mTc- tetrofosmin radiopharmaceutical (Siemens C CAM Soft Corridor 4 D- SPECT – Single- Photon Emission Computed Tomography). The aim of our study was to exemplify a correlation between multiple non-invasive imagistic methods in assessing cardiac function in patients with coronary syndromes and at the same time to underline the role of a new, less used but feasible method – MCE. In the IHD patient, standard echocardiography showed hypokinesia in anterior territory compared to normal wall motion in the second patient. The bulls-eye chart revealed affected global longitudinal strain in anterior segments in case of the patient with prior MI and normal values for the control patient (Figure 1). Additionally, SPECT revealed reduced myocardial perfusion in ischemic patient compared with control. Perfusion polar map is showed in the upper line (Figure 2). Also, regional motility and systolic wall thickening were analyzed using rest myocardial scintigraphy. Please note that scintigraphy images are rotated 45 degree to the right compared to echocardiography images. For MCE, we used as a contrast agent Sulphur hexafluoride microbubbles enclosed in a phospholipid shell (Sonovue®); the contrast agent was administrated via antecubital vein according to European recommendations for contrast echocardiography5. A special continuous infusion pump from Bracco® was used; the images were acquired and analyzed using Epiq 7 G ultrasound equipment from Philips. Furthermore, following a flash of high acoustic power ultrasound, the replenishment rate was assessed as a surrogate marker of myocardial perfusion by analyzing microbubble re-entry into the myocardial circulation. Quantitative perfusion parameters of resting MCE (peak intensity and time to peak) were analyzed using dedicated Q LAB software. All three standard left ventricular apical views (four-, two-, three-chamber views) were acquired by transthoracic echocardiography; we analyzed cine loops length of minimum 10 seconds with a ROI of 5 square millimeters using motion compensation function. The function used for the perfusion curve linearization was local density random walk wash inwash out function (LDRW WIWO). In the case of IHD patient the mean peak intensity was lower in myocardial tissue with impaired microcirculation (3 Db) compared to normal myocardial tissue (12 Db), difference attributed to the reduction in capillary blood volume. Also, time to peak was higher in myocardial tissue with impaired microcirculation (11 seconds) than in control patient (4 seconds).
Figure 1. Regional and global strain for ischemic patient (left) and for the control patient (right).
Figure 2. Scintigraphy images for ischemic patient (left) and control (right).
DISCUSSION
We evaluated left ventricle (LV) function in two patients using complementary imagistic methods. Results obtained by resting MCE, are concordant with those obtained through other validated imagistic methods, but with limitations regarding image quality and attenuation artifact. Our results are concordant with those from literature; a recent study show that in controls (normal tissue) peak intensity was higher: -16.2 ± 8.6 Db, versus -28.3 ±7.3dB in ischemic patients and time to peak was lower: 6.2±0.5 s in control versus 11.5±0.8 s in ischemic patients4. European Society of Cardiology guidelines on contrast echocardiography recommend MCE in patients with suboptimal ultrasound images at rest in order to increase and improve endocardial visualization and
evaluation of LV structure and function when two or more segments are not well viewed in routine ultrasound examination; to make measurements of LV volumes and ejection fraction with higher accuracy and reproducibility; to confi rm or exclude the echocardiographic diagnosis of structural abnormalities of the LV when an accurate diagnosis is needed (in apical hypertrophic
cardiomyopathy, ventricular noncompaction, apical thrombi, and ventricular pseudoaneurysms). Stress MCE is used when two or more limits of the LV endocardium cannot be very well examined, in order to evaluate ventricular wall motility and thickness at stress or at rest, microperfusion and to increase the exam reliability. In a multicenter European study (PHOENIX) that enrolled 628 patients with intermediate-high probability of coronary artery disease (CAD), Senior et al.6 compared MCE and SPECT using coronary angiography. MCE demonstrated higher sensitivity but lower specificity for CAD detection compared with SPECT. Overall, data combined from all published studies showed 84% sensitivity and 78% specifi city of MCE for CAD detection. In patients without a previous myocardial infarction and normal LV function, MCE proved to be superior to SPECT for the detection of moderate CAD7. Subtle endocardial perfusion defects may not be detected by SPECT because of the low spatial resolution compared with MCE. SPECT may be not accurate in the detection of CAD because of disproportionate septal thickening and false perfusion defects due to partial volume effect. These areas with false-positive perfusion defects detected by SPECT appear to have normal perfusion with MCE8. However, MCE is the only technique that allows immediate simultaneous bedside assessment of wall motion and perfusion and, in this regard, it offers a unique role in the diagnosis of ACS. It is a noninvasive procedure, without irradiation. Disadvantages include limited accuracy of the image according to the ultrasound window, positional artifacts; should not be performed in patients with right-to-left shunts, hemodynamic instability, heart rhythm disorders or severe pulmonary pathology. There is insuffi cient data for its use in pregnant women. Although adverse reactions are rare, it may cause headache, paraesthesia, pruritus, facial hyperemia, abdominal pain, chest discomfort wich can simulate an acute coronary syndrome. Assessment of successful reperfusion has been studied by Janardhanan et al.9, who showed that lowpower MCE early after myocardial infarction (MI) can identify microvascular perfusion dysfunction and this can subsequently predict late recovery of the stunned myocardium. In another study, the same group found that MCE can help in the assessment of adequate collateral fl ow in cases of persistently occluded infarct-related arteries. MCE was the only independent predictor of collateral blood flow after MI10. Patients with a persistent defect in the infarcted area due to unrecovered myocardial perfusion had regional or global systolic dysfunction, while those having normal function and perfusion at rest demonstrated an excellent outcome. Moreover, stress MCE may be used to safely assess prognosis in patients with significant cardiovascular risk factors presenting with chest pain, but a negative 12-h troponin and non-diagnostic ECG. In these patients, a negative stress MCE result predicted an excellent prognosis11. The administration of Sonovue® is safe during resting MCE, none of the subjects showed side effects (toxicity, abnormalities of heart rate or blood pressure).
Future perspectives
We expect advances in contrast microbubbles formulations and software technologies with improved imaging quality and post-processing analysis that can generate bulls eye charts for microperfusion. Microbubbles can be used for high precision local drug delivery, such as thrombolysis; they can also be used as carriers for drugs/substances or for transfer of genetic material being safer than viral vectors for DNA. After all, MCE is a promising method for echocardiographic evaluation and also with great future treatment perspectives (as a non-invasive, precise and targeted treatment delivery tool).
Figure 3. MCE perfusion in ischemic (left) and control patient (right).
CONCLUSIONS
Positive correlations were obtained between kinetic parameters evaluated by echocardiography, strain acquisitions, contrast echocardiography, myocardial scintigraphy. These results are concordant with perfusion status of coronary arteries assessed by angiography. Our MCE results are concordant with literature reviews, bring additional information regarding myocardial
perfusion; also MCE can be used as a complementary technique when myocardial scintigraphy is not available or if it cannot be performed.
Acknowledgements: This work was supported by CREDO Project – ID: 49182, fi nanced by the National Authority of Scientific Research and Innovation, on behalf of the Romanian Ministry of European Fundsthrough the Sectoral Operational Programme “Increasing of Economic Competitiveness”, Priority Axis 2, Operation 2.2.1 (SOP IEC -A2-0.2.2.1-2013-1) cofinanced by the European Regional Development Fund.
Conflict of interests: none declared.
Abbreviations:
ACS – acute coronary syndrome
CAD – coronary artery disease
IHD – ischemic heart disease
LDRW WIWO – local density random walk wash inwash
out function
LV – left ventricle
MCE – myocardial contrast echocardiography
MI – Myocardial infarction
ROI – region of interest
SPECT – Single-Photon Emission Computed Tomography
References:
1. Li X, He S, Zhang YS, Chen Y, He JC. Resting Myocardial Contrast Echocardiography for the Evaluation of Coronary Microcirculation Dysfunction in Patients With Early Coronary Artery Disease. Clinical Cardiology 2016;39:453-8.
2. Karogiannis N, Senior R. Contrast echocardiography for detection of myocardial perfusion abnormalities: A clinical perspective. Herz 2017; 42:287-294.
3. Zoppellaro G, Venneri L, Khattar RS, Li W, Senior R: Simultaneous Assessment of Myocardial Perfusion, Wall Motion, and Deformation during Myocardial Contrast Echocardiography: A Feasibility Study. Echocardiography 2016;33:889-95.
4. Orde S, McLean A: Bedside myocardial perfusion assessment with contrast echocardiography. Critical Care 2016;20:58.
5. Senior R, Becher H, Monaghan M, Agati L, Zamorano J, Vanoverschelde JL, Nihoyannopoulos P: Contrast echocardiography: evidence-based recommendations by European Association of Echocardiography. European journal of echocardiography: the journal of the Working Group on Echocardiography of the European Society of Cardiology 2009;10:194-212.
6. Senior R, Moreo A, Gaibazzi N, Agati L, Tiemann K, Shivalkar B, von Bardeleben S, Galiuto L, Lardoux H, Trocino G, Carrió I, Le Guludec D, Sambuceti G, Becher H, Colonna P, Ten Cate F, Bramucci E, Cohen A, Bezante G, Aggeli C, Kasprzak JD. Comparison of sulphur hexafluoride (Sonovue)- enhanced myocardial contrast echocardiography with gated single-photon emission computed tomography for detection of significant coronary artery disease: a large European multicenter study. JAm Coll Cardiol 2013;62(15):1353–1361
7. Senior R, Lepper W, Pasquet A, Chung G, Hoffman R, Vanoverschelde JL, Cerqueira M, Kaul S. Myocardial perfusion assessment in patients with medium probability of coronary artery disease and no prior myocardial infarction: a comparison of myocardial contrast echocardiography with 99mTc single photon emission tomography. AmHeart J 2004;147:1100–1105
8. Hayat SA, Dwivedi G, Jacobsen A, Lim TK, Kinsey C, Senior R. Effects of left bundle-branch block on cardiac structure, perfusion and perfusion reserve: implications for myocardial contrast echocardiography versus radionuclide perfusion imaging for detection of coronary artery disease. Circulation 2008;117(14):1832–1841
9. Janardhanan R, Swinburn JM, Greaves K, Senior R .Usefulness of myocardial contrast echocardiography using low-power continuous imaging early after acute myocardial infarction to predict late functional left ventricular recovery. Am J Cardiol 2003;92(5):493–497.
10. Janardhanan R, Burden L, Senior R .Usefulness of myocardial contrast echocardiography in predicting collateral blood fl ow in the presence of a persistently occluded acute myocardial infarction-related coronary artery. Am J Cardiol 2004; 93(10):1207–1201.
11. Rotaru L, Nanea T. Assessment of myocardial perfusion using contrast echocardiography – Case report. J Med Life. 2015 Oct-Dec; 8(4): 471–475.
 This work is licensed under a
This work is licensed under a