Irina Macovei1, Ana Maria Urluescu1, Iulia Kulcsar1, Marin Postu1, Ioan Mircea Coman1,2, Mihaela Rugina1
1 Emergency Institute for Cardiovascular Diseases ”Prof. Dr. C. C. Iliescu”, Bucharest, Romania
2 University of Medicine and Pharmacy ”Carol Davila”, Bucharest, Romania
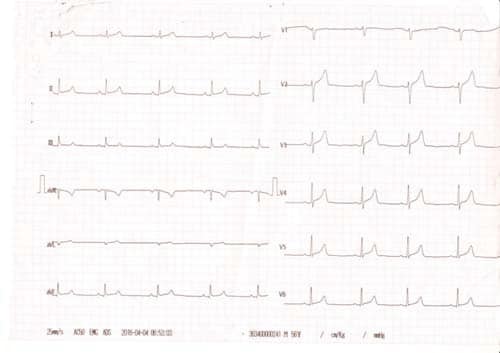
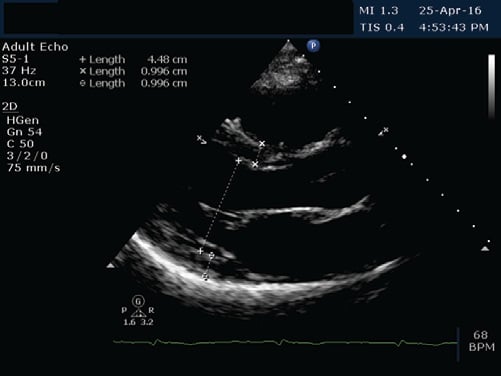
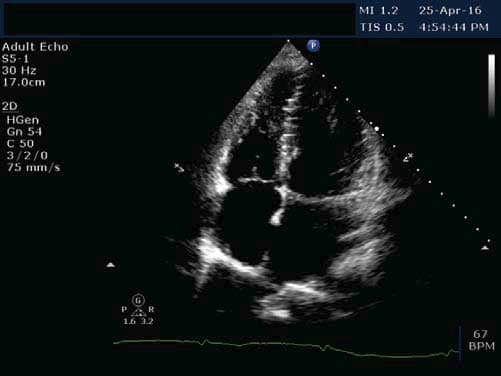
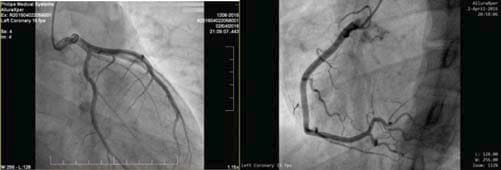
We present the case of a young 20-year-old smoker (5 packs / year), alcohol drinker (weekly) and consumer of psychotropic substances – affirmative Ecstasy, Amphetamine and Cannabis (since approximately 1 year and a half) with a history of a respiratory interference two weeks prior (manifested with fever, chills and myalgia and treated with Cefuroxime 500 mg/day for 5 days) and a recent history of sustained physical activity (two consecutive days of weight lifting in the gym), who presented to the Emergency Department for fast and regular palpitations, dyspnea and chest tightness, symptoms that suddenly begun during the day, after high caffeine consumption. On presentation the patient was haemodynamically stable, BP = 130/80 mmHg, HR = 83 bpm, rhythmic heartbeats, without cardiac or vascular murmurs, no signs of systemic and pulmonary congestion, vesicular murmur preserved. Electrocardiogram (Figure 1) indicates sinus rhythm, QRS spindle at + 30°, ST segment elevation in precordial derivatives (max. 1 mm) and limb derivations (0.5 mm) with early repolarization aspect. The electrocardiogram showed sinus rhythm, QRS axis at + 30° with ST elevation in precordial leads (max. 1 mm) and limb leads (0.5 mm) with early repolarization pattern (Figure 1). Blood samples from the fi rst day of hospitalization showed a normal NT-proBNP level, negative D-dimers, positive troponin (0.067 pg/ml). Also high levels of CK (22.394 u/l), CK-MB (216 u/l) were detected, as well as an important hepatic cytolysis syndrome (TGO = 302u / l, TGP = 89 u/l). Leukocytosis (WBC = 12.845 u/l) with infl ammatory markers (VSH = 44 / mm, CRP = 27.4 ng/ml, fibrinogen = 549 mg/dl) was present, but with negative procalcitonin. Also, a urine sample was tested for the presence of drugs and it tested positive for Tetrahydrocannabinol, the principal psychoactive constituent of cannabis. The transthoracic echocardiography revealed normal- size chambers, preserved left ventricular ejection fraction, mild mitral regurgitation, a free pericardium and spontaneous contrast in the left atrium with an aberrant tendinous chord inserted in the interventricular septum (Figure 2 and 3). Because in the Emergency Department the patient presented with chest tightness, the cardiac markers of myocardial infarction were positive, it was decided to perform a coronarography that showed permeable epicardial coronary arteries with a muscle bridge at the level of LAD with a 40 achieves 40% systolic compression (Figure 4). In all the presented context, because at the guard room the patient has previous thoracic pain, myocardial necrosis enzyme growth, it is decided to perform the coronarography showing permeable epicardial coronary with a muscle bridge at the level of the LAD with a 40% systolic compression (Figure 4). Thus, we have the case of a patient with a recent history of chest pain, with an increase in myocardial necrosis enzymes, with an important inflammatory syndrome and a history of recent respiratory infection. Also to be noted is his history of recent consumption of pshyhoactive drugs and a recent sustained physical activity with a normal coronary angiography. Given the background, the suspicion of myocarditis arises. The etiology of myocarditis is vast, including viral infections (Adenovirus and Parvovirus B19 being the most common pathogens), autoimmune diseases, toxic induced, and drug-related diseases. The presence of a viral infection with cardiac tropism was investigated using PCR for quantitative detection of: Epstein Barr Virus DNA, Herpes Simplex Virus 1 and 2- DNA, Parvovirus- DNA and Cytomegalovirus- DNA), of which a low CMV- DNA titer (1171 copies / ml) was detected. Viral markers for Hepatitis B and C and HIV were negative and tests for thrombophilia factors were negative. Blood samples tested positive for the presence of myoglobin, with an elevated level (850 ng / ml, 10 times over the upper limit of normal). Under medication with beta-blocker, angiotensin converting enzyme inhibitors, antiplatelet, aldosterone antagonist, LMWH, gastric and hepatic protectors the patient’s clinical and paraclinical evolution was slow. A 10-fold decrease until normalization of CK-MB levels was noted and also the levels of hepatic enzymes and infl ammatory markers within normal limits were obtained. Anticoagulation was decided due to spontaneous contrast in the left atrium. Since the patient did not show signs of acute heart failure and evolution was favorable, no myocardial biopsy was performed due to both increased invasiveness and the risk of complications – including the increase of CK and myoglobin in a patient who already had abnormally high levels. A cardiac magnetic resonance imaging (CMR), a useful diagnostic tool for detecting myocardial lesions and to assess left ventricular systolic function was performed in order to confirm the diagnostic of myocarditis. The CMR examination did not reveal areas of fibrosis or myocardial scarring with late gadolinium enhancement, left and right ventricles with normal systolic volume and function (Figures 5 and 6). Thus, CMR did not prove its usefulness, probably because it has not been performed in a timely manner to evidence myocardial edema, the recommendations being that it is done within the first 7-14 days after the onset of the symptoms.
Figure 1. ECG= sinus rhythm, QRS spindle at + 30 °, ST segment elevation in precordial derivatives (max. 1 mm) and limb derivations (0.5 mm) with early repolarization aspect.
Figure 2. TTE, parasternal long axis view, normal dimension heart chambers, no hypertrophy of LV walls.
Figure 3. TEE, apical 4 chamber view, free, normal size chambers.
Figure 4. Coronarography normal coronary arteries.
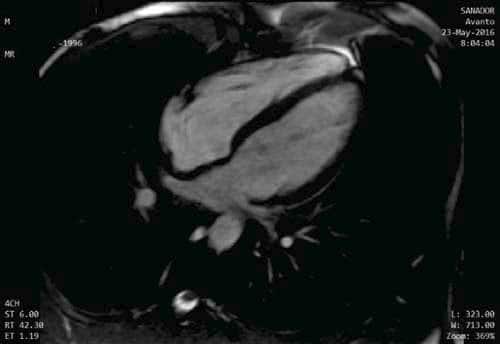
Figure 5. CMR, 4 Chamber View, normal sized heart chambers.
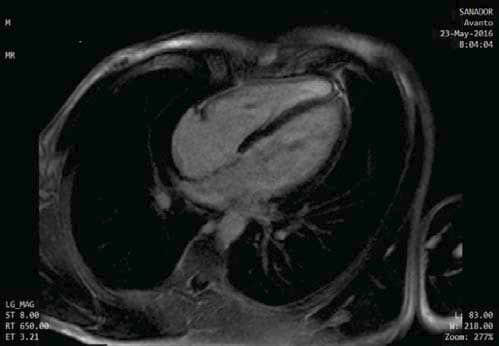
Figure 6. CMR, Late Gadolinium Enhancement, No areas of fibrosis or myocardial edema.
DISCUSSIONS
We are discussing the case of a young patient with a high suspicion of myocarditis, but we cannot ignore the possibility of a rhabdomyolysis. The criteria that suggested a potential rhabdomyolysis are: recent physical activity, consumption of psychoactive drugs and a recent respiratory infection manifested with fever, chills and myalgias, increased levels of CK, CK-MB and myoglobin.
Rabdomyolysis is a syndrome caused by injury to skeletal muscle and involves leakage of large quantities of potentially toxic intracellular contents into plasma. After muscle injury, massive plasma myoglobin levels exceed protein binding (of haptoglobin) and can precipitate in glomerular filtrate. Excess myoglobin may thus cause renal tubular obstruction, direct nephrotoxicity (ischemia and tubular injury), intrarenal vasoconstriction, and acute kidney injury. The most common causes are traumas and muscle compression. Ethanol, methanol, heroin, cocaine, amphetamines, ecstasy and LSD consumption were also found to cause rhabdomyolysis. Also some viruses have been linked to rhabdomyolysis, either by direct effect on the skeletal muscle or through a specific muscle toxin. In adults, only 5% of viral infections lead to rhabdomyolysis, the most common viral agents being influenza A and B, HIV, Coxsackie, Epstein-Barr, cytomegalovirus, Herpes Simplex Virus or Varicella- Zoster Virus. The diagnosis of rhabdomyolysis is clinically suggested by the presence of myalgia, general weakness and hypochromic urine. Elevated serum levels of CK (the
most accurate indicator of muscle injury) and myoglobin are found in patients with rhabdomyolysis. Also increased urinary levels of myoglobin are detected. Myoglobin is a protein present in skeletal and myocardial muscle fibers, with a role in oxygen binding. Its release into the bloodstream is only present in case of muscular damage, so its detection in blood is always pathological. Complications of rhabdomyolysis include electrolyte abnormalities, hypoalbuminemia, hyperuricemia, compartment syndrome, acute kidney injury and renal failure, disseminated intravascular coagulation (a late complication). It can produce acute renal failure in 30% of cases. In the case of the patient presented in this article, the much higher elevation of CK level compared to the CK-MB increase raised the suspicion of a rhabdomyolysis, given the history of sustained physical effort, alcohol consumption and psychotropic substances, CMV infection. But the patient did not show signs or lab abnormalities suggesting kidney damage or electrolyte imbalance during hospitalization and that is why we considered him as suffering rather a myopathy- a muscular impairment without systemic repercussions. Creatin Kinase (CK) is mainly found in the mitochondria and cytoplasm of the skeletal muscle, but also in the myocardium, brain and other visceral tissues. Its main function is in the generation and facilitation of transportation of high-energy phosphates. CK is a dimeric molecule, composed of M and B subunits. The 2 subunits can form 3 isozymes: CK-MM, CK-MB, and CK-BB. Skeletal muscle, myocardium, and neuronal tissue are the main sources of CK-MM, CK-MB, and CK-BB, respectively. The CK-MB isoform is more specific for the heart muscle and is suggestive of acute myocardial infarction, myocarditis, and cardiac trauma (including endomyocardial biopsy). CK elevation is most commonly found in neuromuscular diseases, rhabdomyolysis, and toxic myopathy. CK can also be increased in the absence of neuromuscular disease or cardiac injury, such as sustained exercise or intramuscular injections. In addition, a study of 230 participants who performed 50 flexions of the elbow showed that at 4 days after exercise, 55% had CK > 2000 IU levels, 25% > 10000 IU and 13% > 20000 IU without evidence of kidney injury. Furthermore, there is no clear consensus on the increase in CK levels that can cause rhabdomyolysis. Thus, the predominant increase in CK levels suggests an involvement of skeletal muscle. Cytomegalovirus infection can affect any organ, especially the liver, lung, brain, small intestine or thick intestine. Rarely, cytomegalovirus infection can cause meningoencephalitis, myocarditis or pericarditis. CMV, in immunocompetent individuals, mostly causes an asymptomatic infection or with fl u-like symptoms. CMV hepatitis is accompanied by an increase in liver enzymes accompanied by increases in bilirubin levels. In the patient presented in this article, hepatic enzyme elevation was interpreted as secondary to CMV infection and also the use of psychoactive drugs. However, because of the low detection of CMV-DNA and the hepatic toxicity of antiviral therapy in a patient with increased transaminases, it was thought best not to treat with antiviral therapy. Increased CK MB levels along with troponin elevation and the presence of inflammation markers with normal coronary arteries in a patient with a recent history of respiratory infection and the confirmation of a viral infection with cardiac tropism with Cytomegalovirus can be interpreted as an episode of acute viral myocarditis. On the other hand, marked elevation of CK levels, accompanied by an increase in serum myoglobin levels, can be explained by a number of factors: marked physical activity prior to admission, consumption of psychotropic substances proven to produce muscle cytolysis, the presence of a viral infection with CMV that can affect the skeletal muscle – but all of these elements do not meet the criteria for rhabdomyolysis, but rather for a myopathy. To diagnose with high certainty myocarditis or rhabdomyolysis or both pathologies in a patient with multiple risk factors and symptoms that combine elements from each disease is rather difficult-yet with a favorable evolution under treatment, without systolic dysfunction of the left ventricle or damage to other organs.
Conflict of interest: none declared.
References:
1. Magnani, J. W. & Dec, G. W. Myocarditis: current trends in diagnosis and treatment. Circulation 113, 876–890 (2006).
2. Matthias GF, Marcotte F. Cardiac Magnetic Resonance Assessment of Myocarditis Circulation: Cardiovascular Imaging. 2013; 6: 833-839.
3. Monney PA, Sekhri N et al. Acute myocarditis presenting as acute coronary syndrome: role of early cardiac magnetic resonance in its diagnosis. Heart. 2011 Aug; 97(16):1312-8.
4. Friedrich MG, Sechtem U, Schulz-Menger J, et al. Cardiovascular Magnetic Resonance in Myocarditis: A JACC White Paper. Journal of the American College of Cardiology. 2009;53(17):1475-1487.
5. Matthias GF, Marcotte F. Cardiac Magnetic Resonance Assessment of Myocarditis Circulation: Cardiovascular Imaging. 2013; 6: 833-839.
6. Walker HK, Hurst JW (ed.). Clinical Methods: The history, Physical, and Laboratory Examinations, 3rd edition. Boston: Butterworths; 1990.
7. Latham J, Campbell D, Nichols W. How much can exercise raise creatine kinase level—and does it matter? J Fam Pract. 2008 August; 57(8):545-547.
8. Morandi L, Angelini C, Prelle A, Pini A, Grassi B, Bernardi G. High plasma creatine kinase: review of the literature and proposal for a diag nostic algorithm. Neurol Sci. 2006 Nov. 27(5):303-11.
9. Cervellin G, Comelli I, Lippi G. Rhabdomyolysis: historical background, clinical, diagnostic and therapeutic features. Clin Chem Lab Med. 2010 Jun. 48(6):749-56.
10. Huerta-Alardín AL, Varon J, Marik PE. Bench-to-bedside review: Rhabdomyolysis – an overview for clinicians. Crit Care. 2005 Apr. 9(2):158- 69.
11. Oshima Y. Characteristics of drug-associated rhabdomyolysis: analysis of 8,610 cases reported to the U.S. Food and Drug Administration. Intern Med. 2011. 50(8):845-53.
12. Coco TJ, Klasner AE. Drug-induced rhabdomyolysis. Curr Opin Pediatr. 2004 Apr. 16(2):206-10.
13. David WS. Myoglobinuria. Neurol Clin. 2000 Feb. 18(1):215-43.
14. Cohen JI, Corey GR. Cytomegalovirus infection in the normal host. Medicine (Baltimore). 1985 Mar. 64(2):100-14.
15. Feldman AM, McNamara D. Myocarditis. N Engl J Med. 2000 Nov 9. 343(19):1388-98.
 This work is licensed under a
This work is licensed under a