Ovidiu Stiru1, Lucian F. Dorobanțu1, Alexandra Pasare1, Șerban Bubenek1, Daniela Filipescu1, Horațiu Moldovan1, Vlad. A. Iliescu1
1 Emergency Institute for Cadiovascular Diseases “Prof. Dr. C. C. Iliescu” Bucharest, Romania
Contact address: Ovidiu Stiru, MD. Emergency Institute for Cadiovascular Diseases “Prof. Dr. C. C. Iliescu” Sos. Fundeni 258, sector 2, 022328 Bucharest, Romania Phone/Fax: +40213175227
E-mail: ovidiu_stiru@yahoo.com
Abstract: Objective – Acute aortic dissection (AAD) is the most frequent and catastrophic manifestation of the so-called acute aortic syndrome (along with intramural hematoma, penetrating aortic ulcer, and ruptured thoracic aortic aneurysm)3. The objective of this study was evaluating the cases of acute type A aortic dissection treated in “Prof. Dr. C.C. Iliescu” Emergency Institute for Cardiovascular Diseases over the last nine years, creating a more comprehensive image of this pathology. Material and method – 290 patients (174 male, 116 female, mean age: 55.88±12.13 years) were admitted for acute type A aortic dissection (ATAAD) in our Institute between January 2005 and May 2013, in all cases transesophageal echocardiography being performed for diagnostic confirmation. The main demographic, clinical and perioperative characteristics of these patients were followed and the identification of several factors capable of increasing morbidity and mortality rates associated with acute type A aortic dissection was attempted. Results – The distribution of cases was uniform over time, with a slight decrease of 30-day mortality (p=0.985) and intraoperative mortality rates (p=0.119). Mean age was 55.88±12.13 years and men were more often affected than women (3:2 gender rate). Ascending aorta replacement was the operation performed with the highest frequency (29.71%) and the lowest mortality rate (16.05%) while the highest one associated with ascending, arch and descending aorta replacement (83.3%). 29.10% patients were extubated in the first postoperative day. The most frequent complications were: acute renal failure (61.62%), low ejection fraction ≤30% (57.20%), multiple system organ failure (MSOF) (39.48%), neurologic dysfunction (23.62%). Prolonged cardiopulmonary bypass (CPB) time (over 400 minutes, p=0.001) as well as replacement of the descending aorta (p=0.023) or the aortic arch (p=0.009) associated mortality rates of 80% and 50%, respectively. Conclusion – Type A acute aortic dissection is a frequent pathology in the cardiovascular surgical area. Moreover, it can prove to be a surgical challenge, with increased morbidity and mortality, but in experienced centers the results are more than satisfying. In our experience prolonged CPB time (over 400 minutes) as well as aortic arch and descending aorta replacement significantly increased the.
Keywords: type A acute aortic dissection, surgical treatment, mortality rate.
Rezumat: Obiectiv – Disecţia acută aortică reprezintă cea mai frecventă şi catastrofică entitate a aşa-numitului sindrom aortic acut (alături de hematomul intramural, ulceraţia aortică penetrantă şi anevrismul toracic rupt). Obiectivul acestui studiu a fost evaluarea cazurilor de disecţie acută aortică de tip A tratate în cadrul Institutului de Urgenţă pentru Boli Cardiovasculare “Prof. Dr. C.C. Iliescu” în cursul ultimilor nouă ani, conturând o imagine mai clară a acestei patologii. Material şi metodă – 290 pacienţi (174 bărbaţi, 116 femei, vârsta medie: 55,88 ±12,13 ani) au fost internaţi cu suspiciunea de disecţie acută aortică de tip A în cadrul Institutului în perioada ianuarie 2005 – mai 2013, ecocardiografia transesofagiană (ETE) fiind efectuată pentru confirmarea diagnosticului în toate cazurile. Principalele caracteristici demografice, clinice şi perioperatorii ale acestor pacienţi au fost urmărite, realizându-se un studiu descriptiv, căruia i s-a adăugat o componentă analitică prin identificarea principalilor factori responsabili pentru creşterea ratelor morbidităţii şi mortalităţii asociate. Rezultate – Distribuţia cazurilor a fost uniformă de-a lungul timpului, cu o uşoară scădere a ratelor mortalităţii (p=0.985) şi mortalităţii intraoperatorii (p=0,119). Vârsta medie a lotului a fost de 55,88±12,13 ani, bărbaţii fiind mai des afectaţi decât femeile, cu un raport pe sexe de 3:2. Înlocuirea de aortă ascendentă a fost intervenţia cea mai frecventă (29,71%), care a asociat şi cea mai mică rată a mortalităţii (16.05%), în timp ce la polul opus s-a situat înlocuirea de aortă ascendentă, crosă aortică şi aortă descendentă, cu o mortalitate asociată de 83,3%. 29,10% dintre pacienţi au fost detubaţi în prima zi postoperator. Cele mai frecvente complicaţii au fost, în ordine descrescătoare: disfuncţia cardiacă (57,20%), insuficiența renală acută (IRA) (61,62%), difuncția sistemică multiplă de organ (DSMO) (39,48%), disfuncţia neurologică (23,62%). Durata prelungită a bypass-ului cardiopulmonar (peste 400 minute, p=0,001) alături de înlocuirea aortei descendente (p=0,023) sau a crosei aortice (p=0,009) au asociat mortalităţi de 80%, respectiv 50%. Concluzii – Disecţia acută aortică de tip A reprezintă o patologie frecventă în sfera chirurgicală cardiovasculară. Deşi frecvent se dovedeşte a fi o provocare chirurgicală, cu rate crescute de morbiditate şi mortalitate, în centrele experimentate rezultatele tratamentului sunt mai mult decât satisfăcătoare. În experienţa noastră, durata crescută a bypass-ului cardiopulmonar (peste 400 minute) şi înlocuirea de aortă descendentă sau crosă s-au asociat semnificativ statistic cu creşterea mortalităţii.
Cuvinte cheie: disecţia acută aortică de tip A, tratament chirurgical, mortalitate.
Background
Acute aortic syndrome (AAS) is a collective term for several life-threatening acute aortic conditions1 with similar presentations2: aortic dissection, intramural haematoma (IMH), penetrating atherosclerotic ulcer and traumatic transection1, of which acute aortic dissection is the most frequent and catastrophic manifestation, with a reported incidence of no less than 30 cases per million individuals per year3. In its natural evolution ATAAD associates a mortality rate of about 1% per hour initially, 50% by the end of the third day and almost 80% by the end of the second week. Much lower, although still significant are the mortality rates associated with acute type B aortic dissection (ATBAD): 10% at 30 days, reaching values higher than 70% in the highest-risk groups3. Diagnostic delay is frequent, increased by a wide spectrum of presentations that do not evoke clinical suspicion, and adversely affects outcome2. In these cases the ‘gold standard’ investigation is transesophageal echocardiography (TEE), ideally performed with the cardiac surgical team standing by1. After diagnosis establishment, decisions regarding the initial management, transfer, indication and timing of surgery, and intervention for malperfusion complications are mandatory. The surgical treatment of ATAAD has not been yet subjected to any randomized trials, but novel therapies – particularly with regard to extent of surgery—are being devised and implemented, especially in the treatment of ATBAD2, which is rather different. It has been proven that in the absence of complications optimal medical therapy of ATBAD is reportedly yielding an impressively low 30-day mortality rate of 10% or less. On the other hand, patients presenting with complicated type B dissection are at substantial risk of death or major sequelae; in their case a surgical or endovascular intervention must be considered3. Overall, except in highly specialized centers, surgical outcomes might be static, and there is abundant room for improvement2.
Material and methods
Patients
“Prof. Dr. C.C. Iliescu” Emergency Institute for Cardiovascular Diseases database was queried to identify all patients who underwent surgery for aortic dissection repair between January 2005 and May 2013. A total of 290 patients who underwent repair for acute type A aortic dissections during this period of time were identified (116 female, 174 male, mean age 55.88 years). Patients who did not undergo surgery were excluded from the study. In all cases TEE was performed for diagnosis confirmation in ICU department or on the operating table. All demographic and procedural data and perioperative features of the patients were collected and introduced in an Excel database. 31 variables were defined, SPSS v 16.0 and univariate analysis were used for statistical analysis and a transverse retrospective study was created.
Definitions
Acute type A aortic dissection was defined as any surgically confirmed dissection process that involved the ascending aorta, presenting within 2 weeks from symptoms onset. Cerebrovascular accident was defined as any central neurological deficit persisting for more than 24 h, with CT or MRI confirmation. Chronic renal insufficiency was defined according to estimated glomerular filtration rate. Diabetes was defined as a history of diabetes mellitus, regardless of the duration or treatment of the disease. Acute renal failure was defined as one or both of the following: either a sudden increase in the serum creatinine levels over 2.0 mg/dl or a new requirement for dialysis, postoperatively. Operative mortality included all deaths occurring in the admission period during which the operation had been performed, regardless of its duration, as well as those occurring after hospital discharge, but within 30 days of the procedure.
Operative technique
The acces was performed through a standard median sternotomy. Total cardiopulmonary bypass (CBP) was established in most cases by arterial cannulation of the femoral or right axillary artery and venous cannulation of the right atrium. Myocardial protection was ensured through antegrade or retrograde cold blood cardioplegia administration. A vent was placed in the left ventricle via the right superior pulmonary vein. After a transverse aortotomy, the ascending aorta and the aortic valve were inspected and the segment including the proximal entry tear was resected, followed by repair or replacement of the aortic valve, when needed and replacement of the ascending aorta. After reaching a mean temperature of 25-28°C, the aortic clamp was removed and the aortic arch examined and replaced whenever an arch tear would be identified. The distal anastomosis with prosthetic collagen impregnated low porosity graft was then completed under circulatory arrest with antegrade selective cerebral perfusion. Biological glue and Teflon strips were used in all patients for reinforcement of both proximal and distal suture lines.
Results
290 patients were operated in “Prof. Dr. C. C. Iliescu” Emergency Institute for Cardiovascular Diseases between January 2005 and May 2013, the cases being homogenously distributed throughout the years. Preoperative patient characteristics are summarized in Table 1. The mean age of the lot was 55.88 ± 12.13 years (minimum 20, maximum 96 years), with a slight male predominance (1.5 – gender rate) which became greater for the first two age groups (out of three of 20 years interval defined): 3 in patients under 40 years of age and 1.84 in those between 40 and 60 years old, respectively. Among associated comorbidities, primary arterial hypertension prevailed (75%). In all cases transesophageal ecocardiography was performed for diagnostic confirmation in the ICU department or in the operating room (OR).
Table 1. Preoperative patient characteristics
| Characteristic | Frequency | |
| Age | Male | Female |
| Mean (55.88 years) | ||
| 20-40 years | 75% | 25% |
| 41-60 years | 65% | 35% |
| 61-80 years | 48.60% | 51.40% |
| Gender | 60% | 40% |
| Associated comorbidities | ||
| Arterial hypertension | 90.40% | |
| Bicuspid aortic valve | 8.90% | |
| Marfan Syndrome | 8.10% | |
| Diabetes mellitus | 5.80% | |
| Annuloarctic ectasia | 2.60% | |
| History of myocardial infarction | 2.10% | |
| Previous surgery on the thoracic aorta | 2.10% | |
| Imagistic investigations | ||
| Transesophageal echocardiography | 100% | |
| Contrast CT | 53.90% | |
| Coronary angiography | 3.10% | |
| Aortography | 2.60% | |
| MRI | 1.05% | |
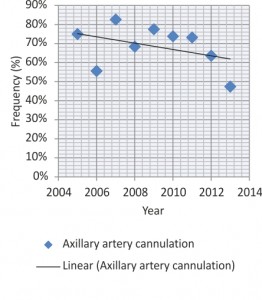
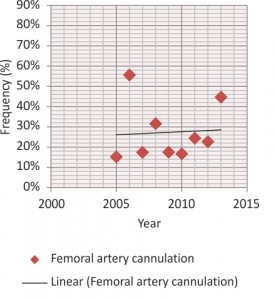
The most used site for arterial cannulation, with a decreasing frequency over time, was the right axillary artery (65.95%), immediately followed by right or left femoral artery (22.94%) (Figures 1, 2). In all cases the entry point was removed, thus performing the replacement of one or more aortic segments involved: an isolated aortic replacement was most often necessary (29.71%), accompanied by hemiarch (34.8%), arch (13.8%) and descending aorta replacement (4.8%). The aortic root was involved in 37.6% cases, repaired in almost three quarters of them through sinotubular junction recalibration, comissures resuspension, David or Yacoub techniques and in one quarter replaced, either isolated or as part of a Bentall operation. Operative patient features are summarized in Tables 2 and 3. Circulatory arrest with moderate or deep hypothermia was performed in all cases for tear inspection of the aortic arch. The average CPB time was significantly longer in operations involving the aortic arch and the descending aorta (452 min) compared to those with isolated replacement of the ascending aorta (172 min). Selective antegrade cerebral perfusion was used in all cases of arch or hemiarch replacement.
Table 2. Operative patient characteristics
| Variable | Frequency (%) |
| Arterial cannulation site | |
| Right axillary artery | 65.95% |
| Femoral artery | 22.94% |
| Left axillary artery | 2.87% |
| Ascending aorta | 2.51% |
| Right subclavian artery | 1.79% |
| Right axillary artery, femoral artery | 1.79% |
| Aortic arch | 0.72% |
| Ascending aorta, femoral artery | 0.36% |
| Aortic valve procedure | |
| Sinotubular junction recalibration | 45% |
| Bentall | 29% |
| Comissure resuspension | 17% |
| Yacoub | 5% |
| David | 2% |
| Replacement | 2% |
Table 3. Operative patient characteristics
| Operations performed | Frequency (%) | Mortality rate (%) | Cerebral perfusion (%) | CPB time (min) | Cross-clamp time (min) |
| Ascending aorta | 29.71% | 16.05% | 6.61% | 172 | 99 |
| Ascending aorta and hemiarch | 23.55% | 27.27% | 38% | 258 | 125 |
| Aortic root and ascending aorta | 22.46% | 25.4% | 4.13% | 241 | 167 |
| Aortic root, ascending aorta and hemiarch | 11.96% | 39.39% | 21.50% | 294 | 175 |
| Ascending aorta and arch | 6.88% | 45% | 12.40% | 350 | 198 |
| Aortic root, ascending aorta and arch | 5.07% | 33.33% | 9% | 398 | 273 |
| Descending aorta | 0.12% | 60% | 3% | 285 | 103 |
| Ascending aorta, arch, descending aorta | 0.20% | 83.3% | 5% | 452 | 283 |
| Root, ascending aorta, arch, descending aorta | 0.04% | 0% | 1% | 351 | 284 |
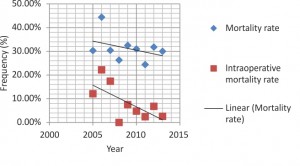
Postoperative characteristics are depicted in Table 4. The average patient was intubated for 23.65 hours and spent 13.6 days in the ICU ward. 29.10% patients were extubated in the first postoperative day. The most frequent complications were: acute renal failure (61.62%), ejection fraction ≤40% (57.20%), MSOF (39.48%), neurologic dysfunction (23.62%). Surgical reexploration was necessary in 26.57% cases, the most common cause being postoperative bleeding. The overall mortality rate was 31.37% (91 patients), with no significant differences in time, while a marked decrease of intraoperative deaths in the last years, although not statistically significant, is visible in the chart below (2005 – 9.09%; 2013 – 1.92%, p=0.119). Greatest mortality rates associated with ascending, arch, descending aorta replacement (83.3%) and with replacement of descending aorta (60%). Related to the replaced aortic segment, mortality rates significantly grew at univariate analysis in descending aorta (p=0.023, OR=3.325, 95%CI: 1.118-9.891) and arch replacement (p=0.009, OR=2.422, 95% CI: 1.227-4.781). Also, while CPB times under 400 minutes associated an overall mortality rate of 24.57%, over this value the mortality rate tripled: 82.46% (p=0.001, OR=15.127, 95% CI: 1.789-127.899).
Table 4. Postoperative characteristics
| Variable | Time |
| Mean ventilatory support period | 23.65 hours |
| Mean length of ICU stay | 13.6 days |
| Complications | Frequency (%) |
| Ejection fraction ≤40% | 57.20% |
| Acute renal failure | 61.62% |
| MSOF | 39.48% |
| Hemodialysis | 36.16% |
| Sepsis | 31% |
| Cerebral stroke | 23.62% |
| Cardiac tamponade | 26.57% |
| Acute myocardial infarction | 9.96% |
| Peripheral ischaemia | 6.27% |
| Visceral ischaemia | 3.69% |
| Mediastinitis | 3.32% |
| Reinterventions | |
| Bleeding | 36% |
| Deep wound infection | 5% |
| Myocardial ischaemia | 2% |
| Tamponade | 2% |
| Peripheral ischaemia | 3% |
| Mortality rate | 31.37% |
Discussion
Acute aortic syndromes (AAS) constitute a spectrum of conditions characterized by disruptions in the integrity of the aortic wall, with potentially catastrophic consequences, including classic aortic dissection, intramural hematoma and penetrating aortic ulcer4, although recent evidences suggest that IMH may in fact be the classic aortic dissection with small intimal tears that are not evident with current aortic imaging techniques5. Of these, acute aortic dissection is the most frequent and catastrophic manifestation1. Traditional classification systems, such as the Stanford and DeBakey, facilitate the decision-making process4; more recently though, a classification based on the pathophysiological features of the aortic lesion rather than its location has been proposed; currently it is recommended that AAD be classified according to both lesion type and location. Still, while the primary goal of surgery is to obliterate the intimal tear in the ascending aorta, thereby preventing flow and encouraging thrombosis of the false lume1, neither of these classification systems dictates the site of the originating entry tear2.
Diagnosis of ATAAD
The estimated total incidence of AAD (type A and B) reaches 30 to 43 cases per million of population per year and apparently continues to increase. ATAAD constitutes more than half of these, while DeBakey type I lesions predominate. It is not known whether the apparent increase in incidence represents improved rates of diagnosis or the dramatic consequence of an aging population2.
While immediate decisions with regard to initial management, transfer, appropriateness of surgery, timing of operation and intervention for malperfusion complications are mandatory, the diverse presentations of ATAAD can delay the diagnosis, adversely affecting outcomes2,6,7. Approximately 75% of patients with acute dissection have their initial diagnosis made in a nonspecialised hospital. In ATAAD, the period of time between presentation and definitive management reaches ~12 h in the majority of patients, but has been reported as long as ~24 h in 20% to 50% of cases in some series8. Symptoms, signs, electrocardiograms and chest X-rays lack sensitivity and specificity, therefore the diagnosis may be overlooked in 40% of cases, sometimes being established only at autopsy8. The primary presentation of AAD to the emergency room (ER) is most commonly an elderly male, with hypertension and sudden onset chest pain8, as it was in our study. On hospital admission, 50% of patients are hemodynamically unstable, ~25% have a neurological deficit, over 20% have cardiac tamponade and 6% have already undergone cardiopulmonary resuscitation9. It has been reported that malperfusion syndromes may be associated in as many as 20-30% of patients presenting with type A dissection; although often under-recognized at that time, they remain responsible for significantly increased mortality rates10.
Without clinical suspicion, patients are not immediately channeled into an appropriate imaging pathway2. Acute aortic syndromes have no reliable point-of-care biomarkers2,8. D-dimer measurements might be for now the most useful tool: a negative assay is highly predictive that a patient does not have dissection, whereas a high level makes the differential diagnosis of ATAAD or pulmonary embolism far more likely and once suspected, definitive imaging comprising CT, transthoracic echocardiography (TTE), transesophageal echocardiography and MRI, is required for confirmation2. TTE is a rapid and readily available investigation in the emergency department and it should be performed without delay in patients with suspected AAS. Owing to an inadequate window for imaging the ascending aorta9, TTE has a reported sensitivity of 59-83% in ATAAD and a much lower one in descending aortic dissection, but a specificity of 63-93% for the diagnosis of aortic dissection1. The sensitivities of MRI, TEE and CT in detecting acute aortic dissection are similar at approximately 95%9. In a comprehensive meta-analysis of all three modalities, the most recent studies reported 100% sensitivity and 100% specificity for TEE, helical CT and MRI, whereas conventional CT (probably the most widely used technique) was less accurate (sensitivity 83-94%, specificity 87-100%). The intimal tear is usually located in the proximal ascending aorta or just distal to the left subclavian artery, measuring less than 5 mm in length – TEE identifies it in about 78-100% of the cases. In another 10-20% of cases, the intimal flap propagates retrogradely, involving the origin of one or both of the coronary arteries1. In this situation, the role of preoperative coronary angiography is still controversial. The low rate of concomitant coronary artery disease, the risk of catheter-induced lesions and management delay are substantial arguments against it. Angiography may be indicated during or after surgery (in the era of the hybrid procedures) in patients with visceral malperfusion and dilatation of the descending aorta for appropriate treatment guidance9.
Surgical indications and predictors of operative mortality
As mentioned above, time-honored dictum is that type A aortic dissection requires urgent surgery. There are, however, controversial situations where the patient’s treatment may stop with medical management: patients with completed stroke, comorbid conditions (e.g., cancer, advanced multiple organ dysfunction, advanced age), prior aortic valve replacement (AVR), and hospital presentation 48 to 72 hours beyond symptoms onset11. The mortality of untreated ATAAD reportedly reaches values of 1% to 2%/h in the first 24 hours, up to 90% of patients succumbing within 30 days. Surgical repair remains high-risk, with both mortality and neurological complication rates of 15% to 30%. No randomized studies of medical vs surgical management in ATAAD have ever been performed, but on the available evidence, surgery converts a 90% mortality rate to at least a 70% survival rate and no more than 2 patients need to be treated to gain survival benefit2. Reported in-hospital mortality for surgically treated cases of ATAAD varies between 5% and 27.4%, reaching values of 28% in the International Registry of Acute Aortic Dissection (IRAD) and 17%, respectively, in the German Registry of Acute Aortic Dissection (GERAADA)9. For patients who present with uncomplicated ATBAD, the survival rate approaches 90% with medical therapy alone, while IRAD reported a mortality rate in patients undergoing surgical repair of 29.3%12. Type II aortic dissection appears to associate a better prognosis than Type I, in terms of perioperative, long-term, and aneurysm-free patient survival, related to the propensity for distal malperfusion phenomena and persistence of a distal false lumen. Arterial hypertension, although considered one of the most important predisposing factors of acute aortic dissection, does not seem to influence the prognosis of these patients13. Preoperative coma, as well as the state of shock secondary to either cardiac tamponade or coronary dissection and ischemia are consistent predictive factors for postoperative mortality2. In a 2012 study Perrault found cardiogenic shock, cerebral ischemia and massive hemorrhage to be responsible for almost 85% of perioperative deaths. According to Pacini et al., the presence of malperfusion tripled the overall mortality rates associated with ATAAD (43.7% in the group with malperfusion vs 15% in that with no malperfusion)10. Another major determinant of outcome in type-A dissection is advanced age. While ATAAD incidence increases with age, recent studies have highlighted the excessive risks associated in patients over 70 years old5. As with all cardiovascular surgery emergencies, advanced age proved an independent predictor of worse operative mortality and morbidity and reduced longer-term survival of ATAAD. However, in western societies one-fifth of the population are 65 years of age and this fraction is increasing2. Mehta and associates have shown that the mortality rate associated with surgery for ATAAD is 45% in patients 80 to 84 years of age and 50% for those 85 or older11. Data from the IRAD registry suggest that one-third of patients presenting with ATAAD are over 70 years of age, with only 47.6% of those older than 80 years of age undergoing surgery. Surgical mortality for this group reaches 40%, compared with 58% for the medically managed patients2. In a 2013 study, Piccardo reported an overall mortality rate in the group of surgically treated octogenarians with ATAAD of 44.3% and identified the presence of complications as the only risk factor for in-hospital mortality. His conclusion was that octogenarians with uncomplicated ATAAD may benefit from emergency surgical repair14. Our study included 11.72% of patients with ages between the range of 70 and 96 years old, without significantly increased mortality rates. Still, the management of acute type A aortic dissection in these age groups remains controversial14.
All these and other factors have been incorporated into predictive risk models on the basis of IRAD and individual center data, which might aid the decision making process. However, although each complication might engender additional risk, this does not preclude a superior outcome with surgery and rigid treatment exclusion criteria are inappropriate2.
*
The aim of ATAAD surgery is prevention of intra-pericardial rupture, of coronary ostial dissection or aortic valve deterioration, with correction of any of these when present, correction of distal malperfusion and permanent obliteration of the distal false lumen (FL). This is usually accomplished by ascending aorta replacement accompanied, where possible, by excision of the proximal entry tear, therefore restoring the dominant true lumen (TL) flow in the distal aorta. Techniques used to achieve these aims have not been subject to randomized studies so they remain issues of debate2.
Arterial cannulation site
The optimal site of arterial cannulation also remains controversial. While femoral artery has been the primary site for arterial cannulation in surgery for ATAAD for a long time2,15, it has lost popularity during recent years given the worse outcomes it appears to associate compared to other strategies in contemporary studies9. Apparently, retrograde perfusion through the femoral artery may further exacerbate dissected intimal flaps and determine organ malperfusion, progressive arch vessel compromise, and neurologic injury and it has also been associated with a greater stroke risk in patients with concurrent distal aortic aneurysmal or atherosclerotic disease2,15. Previous studies have reported an incidence of malperfusion syndrome with femoral cannulation of 2.5% to 13%15. In autopsy series, femoral artery cannulation associated a theoretical potential for brain malperfusion of 42%, whereas with perfusion via the axillary artery this potential was limited to only 16%. However, the clinical incidence of these events is low. Therefore, in ATAAD, initial femoral artery cannulation remains reasonable, if provided malperfusion monitoring is applied and potential distal aortic pathology is excluded2. Besides the decreased risk of stroke or malperfusion, the theoretical advantages of axillary artery cannulation in ascending aorta and arch surgery include the continuous provision of cerebral flow by means of selective antegrade cerebral perfusion. The complications of this technique, such as axillary artery or brachial plexus injury, arm ischaemia and low CPB flow are becoming well-known, ranging between 0% and 5%15. It remains controversial whether the axillary artery should be cannulated directly, or using the sidegraft technique9. In our Institute the primary arterial cannulation site was chosen according to preoperative imaging techniques and intraoperative findings, thus explaining the variable frequencies between the axillary and femoral arteries over the years. Central cannulation is another option, many surgeons preferring to cannulate the dissected aorta itself either with TTE or TEE guidance or under direct vision after transecting the tourniquet controlled distal ascending aorta, therefore allowing very fast cannulation in an emergency. Left ventricular apex is another potential cannulation site7. In a 2013 meta-analysis, axillary artery cannulation seemed to give better short-term mortality and neurological dysfunction rates than femoral artery cannulation16. Because no study was a randomized trial these results are more than uncertain2,16; as the patho-anatomy differs among patients with ATAAD, so does the optimal cannulation strategy9.
Aortic valve involvement
Approximately 30% of patients with ATAAD have an aortic diastolic murmur and one-half have aortic regurgitation on ETT, the surgical management of which is controversial too. Preservation of the native aortic valve has obvious advantages. In case of normal leaflet morphology, of flap interference with valve closure or central regurgitation due to prior dilation of the sino-tubular junction, valve competence can usually be restored by re-affixing the commissures to the aortic wall. However, despite satisfactory early outcomes of this procedure, 20% to 25% of patients will develop late root enlargement or progression of aortic regurgitation, necessitating aortic valve replacement or ARR; risk factors including an aortic annulus of 27 mm in diameter and above-moderate valve regurgitation at initial surgery. In patients with pre-existing root pathology VSRR may be an option, with the added disadvantages of longer operating times and superior technical requirements. Of the 2 types of valve sparing root replacement, the reimplantation technique might be superior in ATAAD2. Kallenbach et al. reported their data after operating on 295 patients for acute type A aortic dissection with use of the supracommissural technique, Bentall procedure and valve-sparing reimplantation technique. In terms of survival and reoperation rates, the authors concluded that the reimplantation technique yields results comparable to those of the established procedures17. Some centers prefer a more aggressive strategy, replacing the aortic valve and adding the well-known risks of prosthetic valves. An aortic root repair (ARR) procedure could also increase risk in inexpert hands. Thus, the role of aggressive ARR management of the aortic valve versus conservative valve re-suspension is incompletely defined2.
In our series aortic valve involvement reached 37.6% of cases. A conservative approach was adopted whenever possible and in only 25% of these cases was the aortic valve replaced either isolated or as part of a Bentall operation. When a competent non-calcified bicuspid aortic valve (BAV) is detected, the decision to conserve will depend upon age, presence of annulo-aortic ectasia and degree of aortic root destruction. If the valve is functionally abnormal, but without associated sinus disease, prophylactic simple aortic valve replacement is justified. Alternatively, reparative bicuspid valve procedures are well-reported, but their application should be judicious2. According to Schäfers et al., the presence of BAV does not influences the outcomes of valve-sparing root replacement (VSRR) procedures. In their series of 153 patients, freedom from reoperation and freedom from recurrent moderate or severe AR were 95% and 90% respectively after 10 years17. The type of disease (i.e. valve insufficiency vs stenosis) influences though the natural history of the aortopathy in BAV patients, as a history of AVR surgery does too18. Also these patients have a distinctive dissection pattern with the entry tear frequently located in the aortic root and-despite their younger age-are at risk of substantial hospital mortality. It has been reported that composite root replacement yields an excellent outcome in BAV patients suffering from aortic dissection, equal to an age- and gender-matched normal population19. The routine use of intra-operative TEE is now regarded as an essential adjunct2.
Extension of aortic repair
In ATAAD the primary intimal tear is usually present within the ascending aorta, sometimes accompanied by secondary, more distal tears. Sometimes, involvement of the ascending aorta can result from retrograde propagation of the dissection flap with the primary tear originating within the arch or descending aorta. Occasionally, no intimal tear is identified2. The aortic arch is dissected in more than 70% of ATAADs. Aortic arch dilatation or obstruction of the supra-aortic vessels is common in ATAAD; luminal arch inspection under DHCA is thus required. The thoracic and abdominal portions of the descending aorta are involved in 40 and 30% of patients respectively, but are responsible for just a minority of acute complications. Therefore, the descending aorta itself is not usually treated during emergency surgery for ATAAD. However, in over 70% of patients the FL is chronically perfused, carrying the risk of further enlargement9. Suboptimal connection of the distal part of the graft implanted in the ascending aorta to the TL or presence of secondary entry tears may account for the postoperative persistence of residual flow into the distal FL. The long-term outcomes of aortic dissections with patent false lumen show a high risk of complications, sudden death and need for surgery, particularly from the third year of evolution. In addition to Marfan syndrome, maximum aortic diameter and the presence of a large, proximal entry tear imply a higher incidence of complications during follow-up20. In the present study an isolated ascending aortic replacement was most often necessary, with an associated mortality rate of 16.05%, combined with a distally extended aorta replacement in 41% of cases. Aortic arch and descending aorta replacement were performed with a frequency of 12.19% and 0.36%, respectively. Both of them significantly increased mortality rates, reaching values of 60% and 83.3%, respectively, once more demonstrating that open aortic arch reconstruction remains a formidable operative procedure, recommended only in highly experienced centers. Probably in association with these complex procedures prolonged CPB time also significantly increased mortality rates: a CPB duration of 400 minutes or longer related to a mortality rate of 82.46%. Recently published results indicate that the dreaded complications of arch repair, namely death and stroke, can occur at rates under 5% in these experienced centers. Himanshu and Deeb reported in a 2013 study on 721 patients with open arch reconstruction a mortality rate of 21.6%21. Novel hybrid techniques have been developed in the treatment of ATAAD with arch involvement. A recent prospective study on the use of hybrid operating rooms for the management of acute dissection demonstrated that a hybrid OR enabled the identification of downstream malperfusion sites, 23% of patients requiring primary endovascular intervention and 35% requiring descending aortic repair in addition to ascending aortic replacement10. The ‘elephant trunk’ is the classical extension of the aortic arch replacement into the descending aorta. During arch replacement, the distal end of the aortic graft is advanced into the descending aorta and connected to the distal aorta in a second procedure. This technique has been refined by the use of stent-graft-reinforced hybrid prostheses which are implanted into the descending aorta in an antegrade fashion during arch replacement (frozen elephant trunk), leading to high false-lumen occlusion rates5,9. However, thoracic endovascular aortic repair (TEVAR), subject of the INSTEAD trial, has become the standard treatment for several descending aorta pathologies. In malperfusion syndromes resulting from dynamic true-lumen compression through a dead-end false lumen, fenestration of the dissection membrane is regarded as a complementary treatment. Today, the method has mostly been replaced by TEVAR9. Operative mortality for acute type A dissection is multifactorial, but remains poorly elucidated10. Although on a descending trend over the last nine years, mortality rates in this study maintain at high levels. Overall, except in highly specialized centers, surgical outcomes might be static, and there is abundant room for improvement2.
Conclusions
Acute type A aortic dissection is a frequent pathology in the cardio-vascular surgical area. Moreover it can prove to be a surgical challenge, with increased morbidity and mortality rates, particularly in extended, complicated forms. The rapidity of diagnostic confirmation and institution of treatment are essential in these cases. Surgical techniques have diversified and improved, especially in the field of aortic valve and root reconstruction and in the field of cerebral protection enabling more extensive aortic arch surgery and lowering morbidity and mortality rates. Endovascular strategies are also emerging, apparently with favorable results. Surgical treatment of ATAAD remains controversial, but in experienced centers the results appear to be more than satisfying.
Note: This work received financial support through the project „CERO – CAREER PROFILE: ROMANIAN RESEARCH”, contract no. HRD / 159 / 1.5 / S / 135760, financed from the European Social Fund through the Sectorial Operational Programme Human Resources Development 2007-2013
Authors’ contributions: All authors have contributed to the manuscript and approved the final version.
References
1. Meredith EL, Masani ND. Echocardiography in the emergency assessment of acute aortic syndromes, European Journal of Echocardiography, 2009; 10: 31–39.
2. Bonser RS., Ranasinghe AM., Loubani M, Evans JD., Thalji NMA., et al. Evidence, Lack of Evidence, Controversy, and Debate in the Provision and Performance of the Surgery of Acute Type A Aortic Dissection, J. Am. Coll. Cardiol., 2011; 58: 2455–2474.
3. Criado FJ, Aortic dissection – a 250-year perspective, Tex Heart Inst J., 2011; 38: 694-700.
4. Bonaca MP, O’Gara PT. Diagnosis and management of acute aortic syndromes: dissection, intramural hematoma, and penetrating aortic ulcer, Curr Cardiol Rep., 2014; 10: 536.
5. Augoustides JGT., FASE, FAHA,* Andritsos M. Innovations in Aortic Disease: The Ascending Aorta and Aortic Arch, Journal of Cardiothoracic and Vascular Anesthesia, 2010; 24: 198-207.
6. Iliescu VA, Dorobantu LF, Stiru O, Chioncel O, Moldovan H, Bubenek-Turconi S, et al. Six years experience in acute aortic dissection type A – retrospective single centre study, 62nd Congress of ESCVS, Regensburg. The Journal of Cardiovascular Surgery. 2013; 54: :3-3.
7. Iliescu VA, Dorobantu L, Stiru O, Bubenek S, Miclea I, Rugina M, et al. Combined cardiac neurosurgical treatment of acute aortic dissection, stroke and coma, Tex Heart Inst J., 2008; 35: 200-202.
8. Ranasinghe AM., Bonser RS. Biomarkers in Acute Aortic Dissection and Other Aortic Syndromes, J. Am. Coll. Cardiol., 2010; 56: 1535–1539.
9. Kruger AT, Conzelmann LO, Bonser RS, Borger MA, Czerny M, et al. Acute aortic dissection type A, British Journal of Surgery, 2012; 99: 1331–1344.
10. Appoo JJ, Pozeg Z. Strategies in the surgical treatment of type A aortic arch dissection, Ann Cardiothorac Surg., 2013; 2: 205-211.
11. Feldman M, Shah M, Elefteriades JA. Medical Management of Acute Type A Aortic Dissection, Ann Thorac Cardiovasc Surg., 2009;15: 286-293.
12. Zheng J, Lu S, Sun X, Hong T, Yang S, et al. Surgical management for acute type A aortic dissection in patients over 70 years-old. Cardiothorac Surg., 2013; 8:78.
13. Apetrei E, Cioranu R, Ginghina C, Coman I, Macarie C. Hypertension does not influence prognosis in aortic dissection, XIIIth World Congress of Cardiology – Free papers, Eds: Monduzzi, Bologna, 1998; 389-393.
14. Piccardo A, Le Guyader A, Regesta T, Gariboldi V, Zannis K, et al. Octogenarians with uncomplicated acute type a aortic dissection benefit from emergency operation, Ann Thorac Surg., 2013; 9: 851-856.
15. Lee HK, Kim GJ, Cho JY, Lee JT, Park I, et al. Comparison of the Outcomes between Axillary and Femoral Artery Cannulation for Acute Type A Aortic Dissection, Korean J Thorac Cardiovasc Surg., 2012; 45: 85-90.
16. Ren Z, Wang Z, Hu R, Wu H, Deng H, et al. Which cannulation (axillary cannulation or femoral cannulation) is better for acute type A aortic dissection repair? A meta-analysis of nine clinical studies, Eur J Cardiothorac first published online July 11, 2014; in press
17. Badiu C, Voss B, Dorfmeister M, Lange R. Valve-Sparing Root Replacement. Where Are the Limits?, Tex Heart Inst J., 2011; 38: 661–662.
18. Etz CD, von Aspern K, Hoyer A, Girrbach FF, Leontyev S, et al. Acute type A aortic dissection: characteristics and outcomes comparing patients with bicuspid versus tricuspid aortic valve, Eur J Cardiothorac Surg., first published online October 15, 2014; ; in press
19. Girdauskas E, Disha K, Borger MA, Kuntze T. Risk of proximal aortic dissection in patients with bicuspid aortic valve: how to address this controversy?, Interact Cardiovasc Thorac Surg., 2014;18: 355-9.
20. Evangelista A, Salas A, Ribera A, Ferreira-González I, Cuellar H, et al. Long-term outcome of aortic dissection with patent false lumen: predictive role of entry tear size and location, Circulation, 2012; 12: 3133-41.
21. Patel HJ, Deeb GM. Open aortic arch reconstruction, Ann Cardiothorac Surg., 2013; 2: 181-183.
 This work is licensed under a
This work is licensed under a