Bogdan Radulescu1,2, Silvia Preda1, Liana Valeanu1, Ruxandra Jurcut1,2, Vlad Anton Iliescu1,2
1 “Prof. Dr. C. C. Iliescu” Emergency Institute for Cardiovascular Diseases, Bucharest, Romania
2 “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania
Abstract: In aortic dissection a tear is made in intima and media and could create a false lumen. This disease is the most lethal of all aortic diseases, with a mortality of 1%/hour without surgical treatment. Neurological complications are more common in type A dissections. Syncope occurs in about 9% of acute cases. Gold standard remains CT scan with i.v. contrast substance and/or TEE and/or MRI. Immediate management of acute aortic dissection includes stabilizing the patient with prompt attention to blood pressure reduction. The purpose of open surgical repair of acute type A aortic dissection is to resect the intimal tear, thereby potentially eliminating entry of blood into the false lumen, reversing collapse of the true lumen, and thus eliminating dynamic obstruction of aortic branches and stabilizing the dissection. We present you a case of a young man with syncope, taken to nearest hospital, where he was diagnosed with ascending aorta hematoma. He was operated as soon as possible, and he was saved, but after a complicated recovery. It is very important to have a rapid and correct diagnosis, to avoid useless therapies and to have an emergent surgical correction. In hospital mortality is more than 30% in patients presenting with shock, cardiac tamponade, hemodynamic instability and unconscious.
Keywords: acute aortic dissection, hematoma, syncope
BACKGROUND
A thorough understanding of aortic anatomy and physiology is necessary for diagnosis and treatment of acute aortic dissection. Aorta is the biggest and most important artery in human body. It runs from the heart to all organs and structures in the body and delivers oxygenated blood. Is divided in three parts: ascending aorta; aortic arch, then descending aorta. Like all arterial structure it has three layers: intima, media and adventitia1-3. In aortic dissection a tear is made in intima and media due to high blood pressure and arterial wall changes, and could create a false lumen, and one or more other tears. This disease is the most lethal of all aortic diseases, with a mortality of 1%/hour without surgical treatment. Between 2,6-3,5 new cases are diagnosed from 100 000 people every year, most of them being men, around the age of sixty1. Main complications are aortic rupture, cardiac failure (aortic regurgitation, acute myocardial infarction, or tamponade), and endorgan ischemia4,2,3. The fi rst attempt of surgical repair was made in 1935, but only in 1955 when De Bakey introduced the transthoracic approach and in 1965 when Wheat adjusted medical treatment, survival was improved5,2. There are three different classifications of aortic dissection, each with its importance: Stanford classification Type A: ascending aorta affected, Type B: ascending aorta not affected; De Bakey classifi cation Type 1: entire aorta affected, Type 2: ascending aorta affected, Type 3: descending aorta affected; and the newest classification Svensson Class 1: classic dissection with true and false lumen, Class 2: intramural hematoma or hemorrhage, Class 3: subtle dissection without hematoma, Class 4: atherosclerotic penetrating ulcer, Class
5: iatrogenic or traumatic dissection. Stanford classification is the most used due to its simplicity and clear surgical indication1,4,2,3. Clinical presentation is very different, from accidentally
discovered, to cardiorespiratory arrest. Usually patients have a severe, acute chest pain (90%), are known with high blood pressure, signs of cardiac tamponade, neurologic manifestation such as stroke (17%), paresis, or digestive ones: abdominal pain, vomiting. Unequal or absent pulses should rise the suspicion of aortic dissection, especially if its related with other cardiovascular symptoms4. Neurological complications are more common in type A dissections. Syncope occurs in about 9% of acute dissection and may be caused by cardiac tamponade, aortic rupture, cerebral vessel obstruction, or activation of cerebral baroreceptors. A particularly dangerous complication of dissection is acute myocardial infarction or coronary ischemia probably due to the dissection flap obstructing coronary fl ow; and this may mask the diagnosis of dissection or lead to inadvertent use of antiplatelet and anticoagulant therapy and delay recognition and treatment of the dissection8. In patients referred to hospital for acute chest pain, differential diagnosis should be made with acute coronary syndrome, aortic dissection, pneumothorax and pulmonary thromboembolism. If the patients have specific electrocardiographic fi ndings and positive cardiac markers for ischemia they can be managed as acute coronary syndrome without further investigations if the medical center doesn’t have the possibility of a transthoracic echocardiography (TTE). But it’s important to continue investigations in patients with unusual symptoms or without specific findings1,9. Considering the complex pathology and management of this lethal disease, diagnosis procedure has some clear goals: confi rm diagnosis, classify the dissection/
delineate the extent, differentiate true and false lumens, localize intimal tear; intimal fl ap, entry sites, distinguish between communicating and non-communicating dissection, side branch involvement (i.e. coronary, carotid, subclavian, celiac, and renal arteries), detect and grade aortic regurgitation, detect extravasations (periaortic or mediastinal hematoma, pleural or pericardial effusion, tamponade)10. If clinical is a high suspicion of acute aortic dissection, a TTE could help, but gold standard remains computer tomographic (CT) scan with i.v. contrast substance
and/or trans esophageal echocardiography (TEE) and/ or magnetic resonance imaging (MRI). A systematic review of the diagnostic accuracy of TEE, CT angiography, and MRI reported a mean sensitivity and specificity of more than 95% for all three investigations4. The choice of imaging modality is influenced by patient characteristics and variables, institutional capabilities and
expertise. Contrast CT is the most commonly used modality. TEE has an advantage in being portable and able to be performed at the bedside for the unstable patient8. Sustained efforts for immediate medical and surgical therapy should start from the moment of diagnosis5. Immediate management of acute aortic dissection includes stabilizing the patient with prompt attention
to blood pressure reduction. Usually beta-blockers are the drugs of choice because of their mechanism of lowering the rate of rise of ventricular force (dP/dt) and stress on the aorta. Intravenous agents are chosen for rapid onset. Emergency surgery is recommended for acute type A. In the International Registry of Acute Aortic Dissection (IRAAD), the mortality rate of patients undergoing surgery for type A dissection was 26% and for those treated medically was 58%. Patients with low-risk features have a signifi cantly lower mortality rate than those with malperfusion, shock, or cardiac tamponade8,10. In-hospital mortality is worst for old patients and those who present in shock. The purpose of open surgical repair of acute type A aortic dissection is to resect the intimal tear, thereby potentially eliminating entry of blood into the false lumen, reversing collapse of the true lumen, and thus eliminating dynamic obstruction of aortic branches and stabilizing the dissection. Despite new devices for minimizing surgical risk, open surgical repair still remains the main treatment for dissection in the ascending aorta, whereas endovascular management is increasingly applied in dissection that is limited to descending parts of the aorta4,10. The aortic valve may need to be replaced, but usually is repaired, depending on the underlying pathology of the valve and aortic root. Also coronary ostium reconstruction is sometimes necessary8,10. The variations of surgical repair depends on preoperative condition of the patient, pathology vaspredisposing to dissection, extent of intimal tear and dissection, state of the aortic root, aortic valve, coronary arteries and not least experience and preferences of the surgeon.
CASE REPORT
A 46 year old white man, known with high blood pressure had syncope during the day and was immediately taken to the nearest hospital. He was not previously hospitalized or investigated. Electrocardiogram (ECG) showed elevated ST segments in V1, V2,V3, and Q wave in V2, V3, but with negative troponin. He was investigated and CT scan with intravenous contrast substance
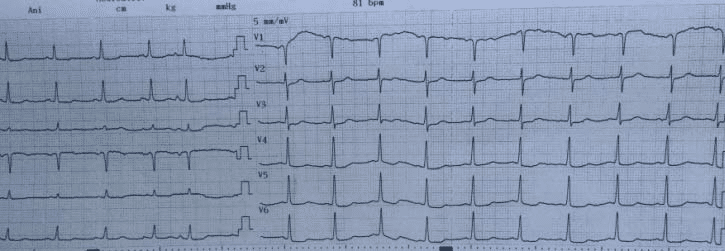
showed a recent small right parietal ischemic stroke, ascending aorta hematoma and important hemopericardium. He was transferred to a tertiary care hospital, in cardiac surgery department for completing investigations and emergency surgical treatment. At admission the patient was unconscious with hemodynamic stability under vasopressor treatment, intubated and ventilated. At our hospital ECG was with no significant pathologic findings as shown in Figure 1. TEE in operating room (OR) revealed a left ventricle (LV) hypertrophy, good left ventricle ejection fraction
(LVEF), moderate aortic valve regurgitation, a dilated ascending aorta with a maximum diameter of 52 mm and important pericardial effusion with a maximum of 26 mm anterior of right ventricle (RV). The patient had standard monitoring, ECG, peripheral oxygen saturation (SpO2), peripheral arterial and venous lines, central venous line, intrarectal temperature, continuous cerebral oxymetry and diuresis. Considering that the patient had an ascending aorta hematoma with no visible false lumen at any level and important pericardial effusion, the surgical strategy was
to isolate femoral artery at the beginning of operation, in case of emergency cannulation.
Figure 1. Admission to our center: ECG with no signifi cant pathologic changes.
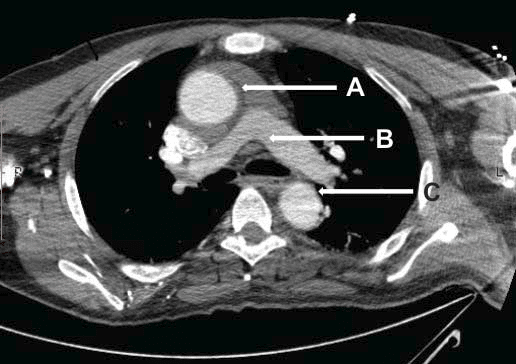
Figure 2. Axial section through ascending aorta showing important intramural hematoma. A. Enlarged ascending aorta with intramural hematoma. B. Pulmonary artery bifurcation. C. Descending aorta.
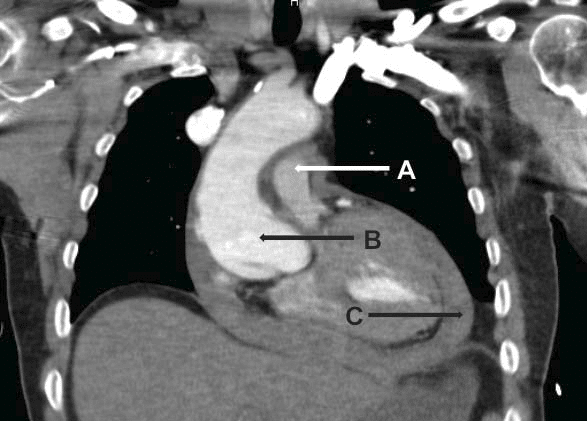
Figure 3. Anterior view of middle mediastinum. A tear is visible in aortic root with intramural hematoma, and important pericardial effusion. A. Pulmonary artery. B. Ascending aorta with intramural hematoma. C. Important pericardial effusion.
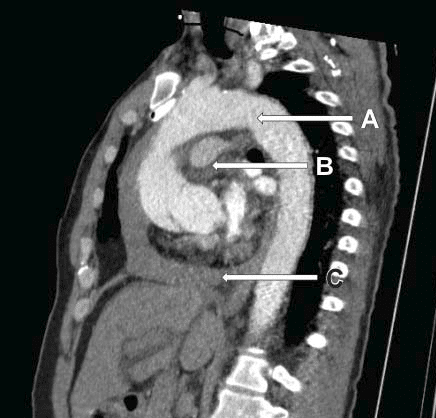
Figure 4. Lateral view showing thoracic aorta, with a tear in aortic root, ascending aorta hematoma, pericardial effusion. A. Thoracic aorta. B. Pulmonary artery. C. Pericardial effusion
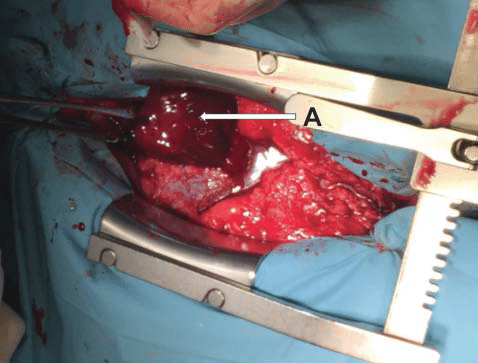
Figure 5. Intraoperative view after pericardiotomy, with evacuation of a large hematoma. A. Intrapericardial hematoma.
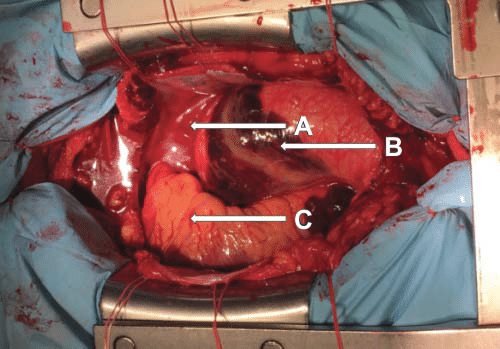
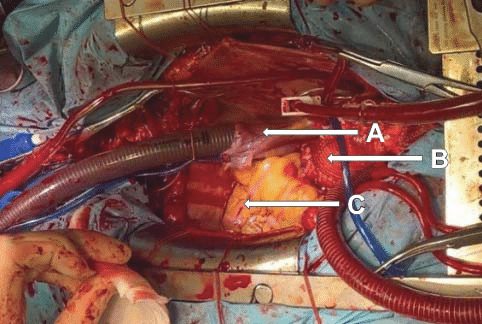
Figure 6. Ascending aorta aneurysm with a parietal hematoma, and the heart moved to the right. A. Right atrium. B. Enlarged ascending aorta with intramural hematoma. C. Right ventricle
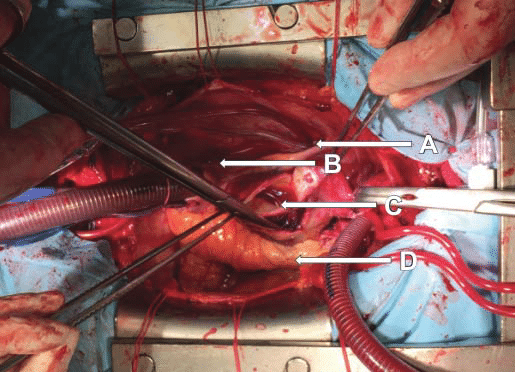
Figure 7. Ascending aorta section, a tear is visible with important parietal hematoma. A. Superior vena cava. B. Right atrium. C. Ascending aorta section: tear visible in non-coronary sinus going circumferentially at about 3 cm above aortic annulus. D. Pulmonary artery.
Figure 8. Ascending aorta section, with a tear in non-coronary sinus and going circumferentially distal. A. Ascending aorta section: tear is visible
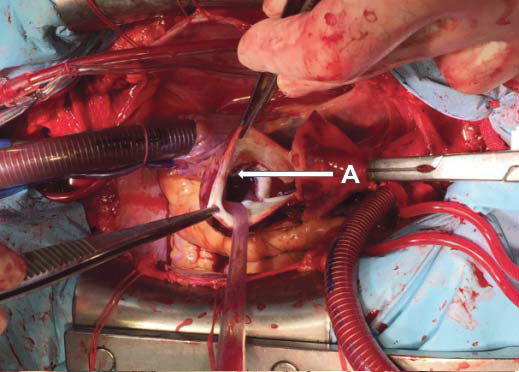
Figure 9. Ascending aorta replacement is complete with a vascular prosthesis. A. Right atrium. B. Vascular prosthesis replacing ascending aorta. C. Right ventricle
The femoral artery was not dissected. Sternotomy and pericardiotomy were made, and a large hematoma was evacuated and an important quantity of blood. The heart had a good contractility, but it was pushed to the left side by the aorta. Ascending aorta had a fusiform dilatation in its proximal half, with a maximum diameter of 80 mm, and a circumferentially parietal hematoma. Fortunately, the diameter was normal before aortic arch, so we decided to cannulate aortic arch.
Cardiopulmonary bypass was established placing an arterial cannula in aortic arch, a two stage cannula in right atrium (RA), venting cannula in LV through right superior pulmonary vein, and a retrograde cardioplegia in coronary sinus through RA. Bretschneider cardioplegia was used. Aortotomy was made with a transverse incision in dilated area of ascending aorta, and a tear was found starting from non-coronary sinus, going circumferentially in ascending aorta at about 3 cm above the annulus. Through this tear a large hematoma was visible. Aortic valve was tricuspid, with normal morphology of the cusps. Both coronary ostium were intact. We excised dilated ascending aorta from sinotubular junction and 8 cm distally for replacing it with a vascular prosthesis number 30. First we did the proximal anastomosis with 3-0 polypropylene running suture, and then the distal one in the same manner. Placing a top cannula in the prosthesis, deairing of the heart was made. After a ventricle electric shock the heart started beating, a slow sinus rhythm, with no ST segment modification and good contractility, so it was slowly weaned off cardiopulmonary bypass with AAI stimulation. In short time afterwards the patient had a decreasing blood pressure, bradycardia with no pace rhythm, diffuse hypokinesia and began fi brillating with no response to electric shocks. He was immediately put on cardiopulmonary bypass and assisted. Inspecting the heart, prosthesis, anastomoses, the only problem found was intense coronary atherosclerosis with multiple calcific plaques in every coronary artery. Because in our center there is no possibility of making coronary angiography in OR, we decided to graft left anterior
descending (LAD) and right coronary arteries (RCA) with left internal mammary artery (LIMA) and saphenous vein graft (SVG). LIMA was harvested in a skeletonized fashion. After clamping ascending aorta prosthesis, anterograde blood cardioplegia was given. Distal anastomosis was done with 8-0 polypropylene running suture, and SVG was implanted in prosthesis with a 6-0 polypropylene running suture in total aorta clamping. The clamp was removed and the heart started. Having the same low sinus rhythm, it was AAI stimulated, but it had better contractility and it was weaned off cardiopulmonary bypass. Despite our efforts and massive blood product transfusions, the patient was opened twice for bleeding. The patient had a recovery time of 25 days in
intensive care unit (ICU) complicated with multiple system organ failure (MSOF), left side hemiparesis, acute respiratory distress syndrome (ARDS), acute kidney injury (AKI) with need of multiple dialysis, hemodynamic vasopressor and inotrope support, severe thrombocytopenia and anemia. Coronary arteries angiography was not possible in OR in our center, and after stabilizing the patient, he and his family refused other invasive investigations until his recovery. He had a good LVEF after operation, with mild RV dysfunction. He had a brain CT scan that didn’t confi rm a new stroke or other changes in the known old one. He also had multiple positive cultures from tracheal secretion, blood, urine, with different multidrug resistant (MDR) bacteria and was treated based on antibiogram and consulting the hospital epidemiologist. Phisiotherapy was started as soon as patients state permited, in ICU and continued in cardiac surgery department and
at home. He was discharged in 36 days from surgical procedure. At first evaluation, in two months from discharge, the patient was in a good clinical state, phisically recovered. Echocardiography showed good contractility, LVEF 50%, no aortic regurgitation, no pericardial or pleural effusion.
DISCUSSIONS
For patients with acute aortic dissection is well understood that coronary angiography can be too dangerous, patients can be too unstable for losing more time until surgical repair, and also this procedure can be undisposable at any hour in some centers. In this particular case we didn’t thought of coronary angiography for multiple reasons like: patient age, hemodynamic state and urgent need of surgical repair. An alternative for not having coronary angiography before surgical correction can be the hybrid operating rooms. This way in every patient with a suspicion of coronary artery lesions can benefi t a fast imaging of vessels and immediately surgical repair.
CONCLUSION
Considering the high mortality and complications of this disease, in every patient presenting with acute onset of chest pain we should think of acute aortic dissection. Every time it is possible a TTE can help in making differential diagnosis, and start treating other more frequent cardiac pathologies, like myocardial infarction, thromboembolism, pericarditis, but only after excluding acute aortic dissection. This is of extreme importance in having the right diagnosis in the shortest time, and in not giving some inappropriate drugs, such as antiplatelet or anticoagulant, and making the
surgery more dangerous and complicated than it already is. It is also of main importance that CT scan is made with i.v. contrast substance, so it will bring all the necessary information like confirming whether the ascending aorta is involved, give information about anatomic features and complications of the dissection, including entry and reentry sites, extent of dissection, branch vessel involvement, aortic regurgitation, pericardial effusion or hemopericardium, and any signs of rupture. All these information are useful for a correct indication and surgical approach, leading to a better survival.
Conflict of interest: none declared.
References
1. Ginghina, C., Mic tratat de Cardiologie, Cap. 30.1, Bolile aortei, Bucharest: Editura Academiei Romane, 2010, pp. 759-766.
2. Cohn, Lawrence H., “Aortic dissection,” in Cardiac surgery in the adult, Elsevier, 2008, pp. 1195-1220.
3. Kilkrin, Barratt-Boyes, Nicholas T. Kouchoukos, Eugene H. Blackstone, Frank L. Hanley, James K. Kirklin, “Acute aortic dissection,” in Cardiac surgery, Elsevier, 2013, pp. 942-967.
4. Jonathan Golledge, Kim A Eagle, “Acute aortic dissection,” Lancet, vol. 372, pp. 55-66, 2008.
5. Coady MA, Rizo JA, Elefteriades JA, “Pathologic variants of thoracic aortic dissection. Penetrating atherosclerotic ulcers and intramural hematomas,” Cardiol Clin, vol. 17, p. 637, 1999.
6. Coady MA, Rizzo JA, Hammond GL,, “Penetrating ulcer of the thoracic aorta: What is it? How do we recognize it? How do we manage it?,” Journal of Vascular Surgery, vol. 27, p. 1006, 1998.
7. Jehad Y. Asfoura, Donald G. Vidt, “Acute aortic dissection,” Chest, vol. 99, pp. 724-729, 1991.
8. Braverman, Alan C., “Acute aortic dissection Clinician Update,” Circulation, vol. 122, pp. 184-188, 2010.
9. Mohammad Mostafa Ansari Ramadi, Mohammad Javad Alemzadeh Ansari, Ata Firoozi, “Acute type A aortic dissection missed as acute coronary syndrome,” Journal of clinical and diagnostic research, vol. 10, no. 5, pp. 33-34, 2016.
10. Ravi Hebballi, Justiaan Swanevelder, “Diagnosis and management of aortic dissection,” Continuing education in anaesthesia, Critical care & pain, vol. 9, pp. 14-18, 2009.
11. Creech O jr, De Bakey ME, Cooley DA, “Surgical treatment of dissecting aneurysm of the aorta,” Texas State J Med, vol. 52, p. 287, 1956.
12. De Bakey ME, McCollum CH, Crawford ES, “Dissection and dissecting aneurysms of the aorta: Twenty year follow up of fi ve hundred twenty seven patients treated surgically,” Surgery, vol. 92, p. 1118, 1982.
 This work is licensed under a
This work is licensed under a