Antoniu Octavian Petris1,2, Loredana Clapa2, Gabriela Sofiniuc2, Mihai Bucatanschi3, Eugen Bitere4, Ioan Lucian Mocanu4, Irina Iuliana Costache1,2
1 “Grigore T. Popa” University of Medicine and Pharmacy, Iasi, Romania
2 “St. Spiridon” County Emergency Hospital, Iasi, Romania
3 “Maycor” Medical Center, Galati, România
4 Clinic of Cardiovascular Surgery, “Prof. Dr. George I. M. Georgescu” Institute of Cardiovascular Diseases, Iasi, Romania
Abstract: Sinus of Valsalva aneurysms (SVA) are rare, mostly congenital, cardiac abnormalities. SVA rupture creates an
aortico-cardiac fi stula, being most common on the right side: right coronary sinus ruptured into right atrium/ventricle. We
report the case of a 28-years-old man which was referred to hospital due to right upper quadrant pain without irradiation
and emphasizing a dynamic dyspnea and fatigue. The clinical examination outlines a right heart failure and the echocardiographic
assessment emphasized the rupture of sinus of Valsalva aneurysm (RSVA) into the right atrium. The anomaly was
corrected surgically with excellent outcome.
Keywords: sinus of Valsalva, rupture, aneurysm, right atrium, echocardiography
INTRODUCTION
SVA can be classifi ed as congenital and acquired: the defects are due to lack of media on the transition area (weakness area) between the fibrous ring of the aortic valve and aortic isthmus. Sometimes aneurysms can coexist with other congenital cardiac malformations such as: a ruptured right sinus of Valsalva into the right ventricle through a ventricular septal defect1, permeable
ductus arteriosus, atrial septal defect, subaortic stenosis and tetralogy of Fallot2-4. SVA had an incidence ranging from 0.1 to 3.5% of all congenital heart defects5, 0.14–0.96% of open heart surgery cases6 and a prevalence of 0.09% in the general population on an autopsy series7. Acquired aneurysms can develop as a result of atherosclerosis, endocarditis, aortic dissection, thoracic trauma, syphilis, Behçet’s disease, Marfan syndrome, Loeys-Dietz syndrome, Ehlers-Danlos syndrome, cystic media necrosis3,4,6,8. Most frequently SVA involve the right coronary sinus (RCS, 65-85%), non-coronarian sinus (10-30%)9,10 and left coronary sinus (below 3%)11. Extracardiac RSVA are rare. Some authors noticed the difference between Asian and Western patients with RASV: in Asian was found a higher RASV incidence, more aneurysms originating from the right coronary sinus, more aneurysms rupture into right ventricle, a higher incidence of association with ventricular septal defect and lower incidence of association with bicuspid aortic valve12. SVA can progress silently for several years and rupture occurs in 3-4 decade of life usually into an adjacent chamber (right heart cavities)13, most commonly into right ventricle (86.7%)14, or into the right atrium, even bicameral rupture into right atrium and right ventricle15, into interventricular septum generating third degree atrioventricular block or into pericard producing tamponade11,16-18. Initially rupture may be silent, but later manifests itself as a progressive heart failure caused by arterio-venous communication and aortic regurgitation19. Sinus of Valsalva aneurysm classifi cation was proposed by Sakakibara and Konno (1962)20:
• Type I: aneurysm has its origin in the left side of the right coronary sinus (RCS) and his rupture is into right ventricle near the pulmonary valve. Usually this type associated ventricular septal defect (VSD) under the pulmonary valve.
• Type II: aneurysm originated in the middle portion of the RCS and his rupture is into right ventricle. VSD is uncommon.
• Type III: aneurysm has its origin in the middle portion of the right coronary leafl et protruding toward the tricuspid valve. The rupture of this type is into right atrium and, sometimes, in right ventricle under the tricuspid valve insertion.
• Type IV: aneurysm originated in the right portion of the non-coronary leafl et and ruptures into the right atrium (RA). The clinical picture can sometimes mimic pulmonary thromboembolism that can be a catastrophic confusion when a unnecessary thrombolytic therapy is administrated.
CASE REPORT
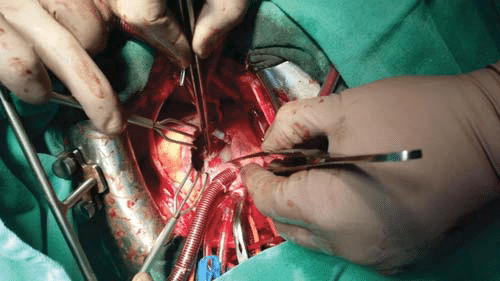
We present the case of a male patient, 28 years old, from rural area, admitted in the Cardiology Clinical, „St. Spiridon” County Hospital Iaşi in April 2015, without a history of cardiovascular pathology, symptomatic since approximately 1 month, with pain in the right upper quadrant without irradiation, small-to-medium exertional dyspnea, afebrile, dyaphoresis and asthenia which have gradually increased. The clinical examination reveals IV/VI degree (Levine) pluriorificial continuous (‘‘to and fro’’) systolic-diastolic murmur, predominantly in the aortic auscultation area, irradiating along the left sternal border, accompanied by a thrill, rounded enlarged liver 3 cm below the rib edge, slightly painful, without declive edema. Should be noted that diastolic accentuation of this “machinery” murmur is an important sign of differentiated RSVA from patent ductus arteriosus2,14, missing in this case. The patient complaints starts just after a sustained physical effort (he was a construction worker) with new onset shortness of breath, fitting a New York Heart Association (NYHA) functional class II. The RSVA initial diagnosis was established by echocardiographic exam as outpatient (“Maycor” Medical Center, Galaţi) and then the patient was sent to our Cardiology Clinic to establish his further therapeutic management. Here we confirmed (by a new transthoracic echocardiography) the previous diagnosis, objectifying the aorta-right atrium shunt (Figure 1) which significant charge the pulmonary circulation, right heart cavity dilatation and pulmonary hypertension (systolic pulmonary arterial pressure, sPAP ~50 mmHg), left atrium diameters 49/63/44 mm, ascendent aorta diameter 30 mm, aortic Vmax 1,6 m/s, Left Ventricular End Diastolic Diameter (LVEDD) 57 mm, Left Ventricular End Systolic Diameter (LVESD) 35 mm, interventricular septum thickness 11 mm, left ventricle walls thickness 11 mm, Left Ventricular Ejection Fraction (LVEF) 67%, SF-38%, RV 34 mm, Right Atrium diameters (RA) 54/52 mm, Right Ventricular End Diastolic Diameter (RVEDD) 21 mm, normal left cardiac cavities, normal segmental and global kinetic, no addition images on valves (Figure 2 and 3), tricuspid aorta with no vegetations or others valvular abnormalities. Asymmetric dilatation of the aortic root can easily be emphasized (specific feature of “windsock” dilatation). Color fl ow Doppler revealed a continuous turbulent flow between right sinus of Valsalva and the right atrium through the ruptured aneurysm. ECG reveals a sinus tachycardia 90 bpm, QRS axis at 45°, normal morphology. A discreet hepatic cytolysis: aspartate aminotransferase (ALAT/TGO) 45/40/40 UI/l, alanine aminotransferase (ALAT/TGP) 148/135/57 UI/l, gamma-glutamyl transpeptidase (GGT) 91/93/112 UI/l, hypoxemia with respiratory alkalosis. Moderate cardiomegaly on chest radiography. Concerning the etiologic diagnosis, it was ruled out syphilis (VDRL negative) and Marfan syndrome (no eyes disorder, normal stature, normal fingers)3,4. Clinical and echocardiographic was also been ruled out other possible malformations associated with ruptured Valsalva aneurysm (aortic insufficiency, aortic stenosis, atrial or ventricular septal defect, coarctation of the aorta). Nor angiography or pulmonary CT angiogram was performed. The patient outcome in our clinic was to haemodynamic rapidly impairement with increasing PASP (70 mmHg) at TTE, low BP, extreme fatigue, increased breathlessness and diaphoresis, symptoms which required the transfer of the patient in surgery ward at Institute of Cardiovascular Diseases from Iaşi to correct the defect. Treatment for this type of pathology is surgical and consists in closing the communication (the defect correction with Dacron-patch – Figure 4 and 5), after external chest hypothermia, anterograd cardioplegia and right atriotomy approach. Operative results, postoperative course and survival are excellent, he was discharged on eleventh postoperative day with the following recommendations: amlodipine 10 mg once daily, bisoprolol 5 mg once daily, aspirin 75 mg once daily, ranitidine 150 mg once daily. Echocardiography finding at discharge are Left Ventricular End Diastolic Diameter (LVEDD) 55 mm, Left Ventricular End Systolic Diameter (LVESD) 40 mm, Interventricular Septum thickness (IVS) 9 mm, thickness of the left ventricular free wall (LVW) 11 mm, Left Ventricular Ejection Fraction (LVEF) 50%, Left Atrium diameter (LA) 56/50 mm, Right Atrium diameter (RA) 49/40 mm, Right Ventricular End Diastolic Diameter (RVEDD) 36 mm, ascendent aorta diameter 37 mm, mitral valve surface area 3.8 cm2, Pressure Half Time (PHT aortic) 58 ms, Aortic vmax 1,1 m/s, dPmax (tricuspid) 20 mmHg, Ist degree mitral regurgitation, Ist degreee aortic regurgitation, Ist degree tricuspid regurgitation, LV global systolic function preserved, free heart cavities, pericardium without fluid, at the level of the synthetic patch between right coronary sinus and right atrium it can be seen a very small residual shunt, hemodynamically insignificant. No signs of pulmonary hypertension.
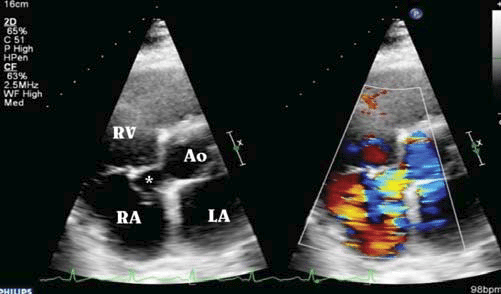
Figure 1. Transthoracic echocardiography (parasternal short axis view, without/with color-fl ow Doppler mode): aneurysmal dilatation of right sinus of Valsalva (*) protruding into the right atrium (RA); aorta-right atrium shunt with right cardiac chamber (RA and RV) dilatation and severe pulmonary hypertension (systolic pulmonary arterial pressure, sPAP ~ 70 mmHg). Ao aorta; LA left atrium.
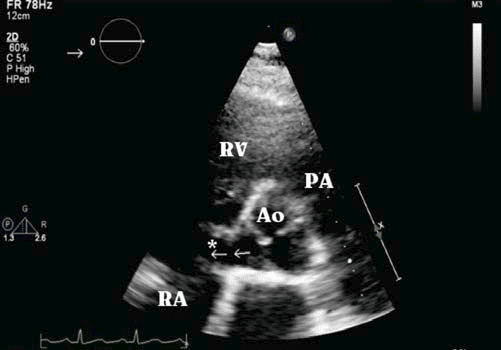
Figure 2. Transthoracic echocardiogram (parasternal short axis view): ruptured aneurysm of noncoronary sinus of Valsalva (*) into the right atrium (RA) with left-to-right systolic-diastolic shunt (¬¬) which loads right atrium, right ventricle (RV) and pulmonary circulation. Ao aorta; PA pulmonary artery.
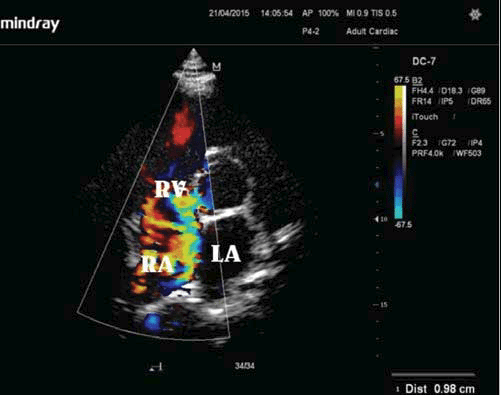
Figure 3. Transthoracic echocardiogram (apical 4-chamber view): ruptured aneurysm of noncoronary sinus of Valsalva into the right atrium which loads right heart circulation.
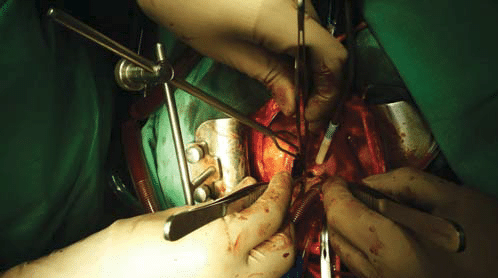
Figure 4. Operative view: noncoronar sinus Valsalva aneurysm ruptured into the right atrium.
Figure 5. Operative view: correction of rupture with Dacron patch.
DISCUSSIONS
In young patients (mostly male – 4:1 ratio – and middleaged), the ruptured aneurysms of sinus of Valsalva may initially be asymptomatic or may be manifested by acute heart failure with the emergence of new heart murmurs imposing a differential diagnosis with other conditions that can cause continuous murmur such as patent ductus arteriosus, aorto-pulmonary window, coronary fistula, ventricular septal defect and aortic regurgitation. SVA without rupture can cause obstruction of the right ventricular outfl ow tract and may be complicated by endocarditis11,18, thrombus formation, embolic events, myocardial infarction (compression of the coronary artery branches by the aneurysm), cardiogenic shock and death21. The symptoms described in patients with unruptured SVA is dominated by medium-small effort dyspneea, palpitations and angina. In addition to the echocardiography, the angiographic study is used to accurately detect the site of the rupture, as well as to estimate the permeability of the coronary vessels. The median diameter of lesions was 6 mm (4-8 mm)22. MRI can be used to confirm the diagnosis23,24, aneurysmal sac position, direction of expansion, the heart cavity in which rupture occured and other coexisting injuries. Approximately a third of patients with RSVA with left-right shunt complain angina, breathless and fatigue, symptoms that worsen progressively requiring further investigation. Electrocardiogram can objectify left/right ventricle hypertrophy and non-specifi c ST segment/ T wave abnormalities. The management of RSVA is guided by clinical and echocardiographical fi ndings. Transesophageal echography is a further examination wich can provide valuables data as compared to with transthoracic echocardiography for the aneurysm and coexisting cardiac malformations description. Due to the coexistence of two systolic jets in the right atrium, the difficulty of measuring PAPs should be mentioned. In fact, in our case report the disappearance of pulmonary arterial hypertension after surgical repair indicated that the component responsive to elevated pre-operative sPAP values was the aortic jet. Additional diagnosis methods include cardiac catheterization, computed tomography angiography and cardiovascular magnetic resonance10. Early diagnosis is important for emergency surgery. Vural et al19 used a simple and functional algorithm of the therapeutic approach to ASVs, based on the clinical picture and the echocardiographic findings: endo cardiac ruptured (type A) or unruptured (asymptomatic – type B-I / symptomatic – type B-II) and extracardiac (type C). Surgical approach is required for both ruptured/ unruptured (but symptomatic) aneurysms. SVA become symptomatic when their size exceeds 50% of the average size of the other two sinuses and when there is a compression of the surrounding tissues25,26. The surgical treatment have a low risk and has a longterm survival rate similar to that of healthy individuals when not coexist other malformations (in a series of 19 cases enrolled between 2009-2013 the hospital mortality rate was 5.2%)27. Surgical correction can be summarized in a simple suture or graft plasty with patch closure of the defect27. Because of RSVA rarity there have been no clinical data to support one or other closure technique (primary vs patch closure) and preferred surgical approach (dual, transaortic, involved chamber), but using a patch, as in our reported case, minimize aortic leafl et distortion and reduces the stress on the suture line20,27. The early surgical treatment can prevent the evolution to congestive heart failure, endocarditis or death. In selected RSVA patients percutaneous closure using modifi ed double-disc occluders can be an alternative to surgery22. The particularity of this case lies in the fact that aneurysm rupture occurred at a young age (28 years) in decade 2-3 of life than the average age cited in the literature (decade 3-4) and without any associated congenital or acquired disease. Our patient have a SVA type III according to the Sakakibara and Konno classifi cation, its origin in the middle portion of the right coronary leaflet, protruding toward the tricuspid valve and the rupture is into right atrium.
CONCLUSIONS
Ruptured sinus Valsalva aneurysms, though rare, can have poor prognosis in the case of delated diagnosis. An important diagnostic contribution brings the transthoracic echocardiography which confirms the diagnosis and allow the fastest possible surgical treatment without which the patient outcome would be potentially life threatening.
Consent: Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal.
Competing interests: The authors declare that they have no competing interests.
References
1. Chatzis A, Kanakis M, Tsoutsinos A, Mitropoulos F. Ruptured sinus of Valsalva: not always an aneurysm. Hellenic J Cardiol 2015; 56: 344- 346.
2. Arikan E, Karagöz A, Bayata S, Yilik L, Ünluer EE. Case report – a rare cause of dyspnea: sudden rupture of aortic Valsalva sinus aneurysm. Case Reports in Medicine 2013 http://dx.doi.org/10.1155/2013/909302
3. Knight JL, Jacka M, Charrette JP. Repair of isolated, symptomatic sinus of Valsalva aneurysm in a patient with Marfan’syndrome. Can J Cardiol 1988; 4: 214-216.
4. Chue SH, Hung CR, How SS, et al. Ruptured aneurysm of the sinus of Valsalva in oriental patients. J Thorac Cardiovasc Surg 1990; 99: 288- 298.
5. Goldberg N, Krasnow N. Sinus of Valsalva Aneurysms, Clin Cardiol 1990, 13: 831– 36.
6. Takach TJ, Reul GJ, Duncan JM, Cooley DA, Livesay JJ, Ott DA, Frazier OH. Sinus of Valsalva aneurysm or fi stula: management and outcome. Ann Thorac Surg 1999; 68: 1573-7.
7. Smith WA. Aneurysm of the sinus of Valsalva, with report of 2 cases. J Am Med Assoc 1914; 62: 1878.
8. Shah RP, Ding ZP, Ng AS, Quek SS. A ten-year review of ruptured sinus of Valsalva: clinico-pathological and echo-Doppler features. Singapore Med J 2001; 42: 473-6.
9. Felsoci M, Svatopluk N, Andrasina T, Nemec P. Ruptured aneurysm of non-coronary sinus of Valsalva as a rare cause of chest pain. Eur Heart J 2014; 35(31): 2123.
10. Abdelsalam M, Bachinsky W, Pawlush D, Mumtaz M, Goldman J. Noncoronary sinus of Valsalva aneurysm rupture into right atrium. Tex Heart Inst J 2013; 40: 493-494.
11. Shumacker Jr HB. Aneurysms of the aortic sinuses of Valsalva due to bacterial endocarditis, with special reference to their operative management. J Thorac Cardiovasc Surg 1972; 63: 896-902.
12. Wang ZJ, Zou CW, Li DC, et al. Surgical repair of sinus of Valsalva aneurysm in Asian patients. Ann Thorac Surg 2007; 84: 156–60.
13. Lakoumentas JA, Bonou MS, Brili S, Theocharis CS, Benroubis AD, Perpinia AS, Harbis PK. Ruptured aneurysm of the right sinus of Valsalva into the right ventricle. Hellenic J Cardiol 2002; 43: 242-245.
14. Chang C-C, Chin C-H, Chen M-L, Chen T-H, Lo H-S. Sinus of Valsalva Aneurysm with Rupturing into the Right Atrium – A Case Report and Review of the Literature. Acta Cardiol Sin 2006; 22: 96-101.
15. Khandeparkar JM, Porwal MM, Agarwal ChU, Mahajan VD. Bicameral rupture of aneurysm of sinus of Valsalva. Ind J Thorac Cardiovasc Surg 2009; 25: 198-200.
16. Van Son J, Danielson GK, Schaff HV, Orszulak TA, Edwards WD, Seward JB. Long-term outcome of surgical repair of ruptured sinus of Valsalva aneurysm. Circulation 1994; 90: 1120-1129.
17. Koh KK, Lee KH, Kim SS, Lee SC, Jin SH, Cho SW. Ruptured aneurysm of the sinus of Valsalva in a patient with Behcet’s disease. Int J Cardiol 1994; 47: 177-179.
18. Biabnam KR, Roberts WC. Fatal intrapericardial rupture of sinus of Valsalva aneurysm. Am Heart J 1990; 120( Pt I ):1455-1456.
19. Vural KM, Sener E, Tasdemir O, Bayazit K. Approach to sinus of Valsalva aneurysms: a review of 53 cases. Eur J Cardio-thorac Surgery 2001; 20: 71-76.
20. Sakakibara S, Konno S. Congenital aneurysm of the sinus of Valsalva: anatomy and classifi cation. Am Heart J 1962; 63: 402-424.
21. Gupta S, Chitlangia R, Garg D. Echocardiographic diagnosis of ruptured sinus of Valsalva with congenital VSD and AR. J Clin Prev Cardiol 2015; 4: 24-6.
22. Liu S, Xu X, Zhao X, Chen F, Bai Y, Li W, Zhang Y, Wang C, Xiang J, Wu G, Chen X, Qin Y. Percutaneous closure of ruptured sinus of Valsalva aneurysm: results from a multicentre experience. EuroIntervention 2014; 10: 505-12.
23. Dev V, Goswami KC, Shrivastava S, Bahl VK, Saxena A. Echocardiographic diagnosis of aneurysm of the sinus of Valsalva. Am Heart J 1993; 126: 930-936.
24. Yylmaz AT, Demirkylyc U, Ozal E, et al. Aneurysm of the sinus of Valsalva. J Cardiovasc Surg 1997; 38: 119-124.
25. Mayer ED, Ruffmann K, Saggau W, Butzmann B, Mayer KB, Schatton N, Schmitz W: Ruptured aneurysms of the sinus of Valsalva. Ann Thorac Surg 1986; 42: 81-85.
26. Tami LF, Turi ZG, Arbulu A. Sinus of Valsalva aneurysms involving both coronary ostia. Cath Cardiovasc Diag 1993; 29:304-308.
27. Yadav A, Mathur R, Devgarha S, Abraham V, Sisodia A. Surgery for ruptured sinus of Valsalva aneurysm: Five-year experience with 19 patients. Turk Gogus Kalp Dama 2014; 22: 729-733.
 This work is licensed under a
This work is licensed under a