Costica Baba1, Mihai Ursu1, Narcis Filipescu1, Diana Tint1,2
1 ICCO Clinics, Brasov, Romania
2 Faculty of Medicine, „Transilvania” University, Brasov, Romania
Abstract: The risk factors for aortic aneurysm usually include history of smoking, chronic obstructive pulmonary disease and hypertension, but patients at highest risk for abdominal aortic aneurysms (AAAs) are those who are older than 65 years and have peripheral atherosclerotic vascular disease. Patients who have first-degree relatives with AAA are also considered as having high risk1. Less frequent causes of AAA are Marfan and Ehlers-Danlos syndromes, collagen vascular diseases, and mycotic aneurysms. AAAs are usually asymptomatic until they expand and disrupt1. Keywords: abdominal aortic aneurysms, hypertension
CASE REPORT
We report the case of a 79 years old male having a recently discovered AAA during a routine abdominal ultrasonography examination. The patient was a non smoker, alcohol abstinent, with history of hypertensi-on, dyslipidemia and atrial fibrillation (AF). The patient complained of light exercise chest pain and fatigue. He was chronicaly administered betablocker, angiotensin converting enzyme inhibitor, statine and oral anticoa-gulation agent.
Clinical examination at hospital admission revealed normal BMI (25 kg/m2), irregular rhythm with heart rate of 74 beats/min, with no pathologic sounds and murmurs. Blood pressure was 140/80 mmHg. Other fi ndings of the physical examination were not clinically signifi cant. Biological parameters were in the normal range. The 12-lead electrocardiogram (ECG) indicated AF with a ventricular rate of 70. Transthoracic echo-cardiography (TTE) documented moderate concentric left ventricular hypertrophy (14 mm), hypokinesia at the level of basal segments of the infero-posterior wall and mild mitral regurgitation. Heart chambers were otherwise normal sized and left ventricular ejec-tion fraction was normal (LVEF 50%). Ultrasonogra-phy examination of the carotid arteries revealed diffu-se atheromatosis with no significant stenosis.
A multi-slice angio-CT targeting the abdominal seg-ment of the aorta and peripheral arteries was perfor-med in order to obtain a better anatomic characteri-sation of the aneurysm and more accurate mesure-ments of the aneurysm and the ilio-femural arteries diameters. The aneurysm was located 5 cm below the renal arteries take-off and had the maximum size of 77/85 mm (Figure 1, 2). The AAA’s neck diameter was 24 mm and proximal neck angulation was <60° with respect to the body of aneurysm. The distal landing zone of aneurysm was si-tuated in the close proximity of the aortic bifurcation. Both iliac and common femoral arteries were calcified but normally sized with no significant tortuosity (Fi-gure 3).
Due to the abnormal movement of the infero-pos-terior wall, a coronary angiogram was performed and revealed a severe stenosis of a dominant left circum-fl ex artery (a 4.5 mm diameter vessel) located next to the origine of the fi rst marginal branch artery (Figure 4).
Having all this data we may conclude that, in this patient, the major clinical issue consists in the presen-ce of a huge infrarenal aortic aneurysm with a very high risk of disruption, in association with a severe coronary lesion to be treated, in the context of a long-life need of chronic anticoagulant therapy due to the association of AF. It is known that the risk of aneurysm rupture increa-ses exponentially with the aneurysm size. The annual risk of rupture is low in AAA having maximal diameter below 5 cm (<1%), the risk of rupture is ~10% in AAA having maximal diameter >5-6 cm, 20% in AAA with the diameter >6 cm and over 30% if the diameter of aneurysm exceeds 8 cm1,2. The case was taken for Heart Team evaluation and we decided to manage the case using mini-invasive techniques.
Firstly, we performed the percutaneous myocardial revascularization. In this respect, a balloon self appo-sing stent of 3.5-4.5/ 22 mm was used in order to treat the severe lesion of the circumfl ex artery. The fi nal angiographic result is depicted in Figure 5.
Second step was undertaken two days later and tar-geted the endovascular aortic repair (EVAR) of the aortic aneurysm. The intervention was performed in the cath-lab under general anesthesia and standard anticoagulation protocol (intravenous heparin targe ting an activated clotting time up to 300 seconds). The team decided to use a bifurcated infrarenal stent graft (AFX- Endologix), a special endoprosthesis which is tailored to be anatomically fixed directly on the aor-tic bifurcation. The right femoral artery was cut-down for access using standard surgical techniques. A 17 Fr sheath was placed inside the surgically prepared right femoral artery in order to advance the delivery system with the aid of a 0.035“ stiff guidwire. Another 7 Fr sheath was inserted into the left femoral artery using percutaneous technique. A snare cathether, introdu-ced through left side femoral artery, served us to fix the system over the aortic bifurcation and to deploy it at the level of left iliac limb. A stentgraft Endolo-gix AFX 2 (BEA 25-100/ I16-40) has been implanted on the aortic bifurcation and the Endologix Vela (A 28-28/ C95-O20V) aortic extension was placed in the infrarenal segment of the abdominal aorta. A 32 mm diameter balloon was used for postdilatation. Deployment of device and the fi nal result is depic-ted in Figure 6. The patient was discharged 4 days later. An ultra-sound examination of the aorta and branches was performed at discharge and revealed a normal tri-phasic flux at the level of both iliac and femoral artery. The angio CT examination performed at one month follow-up showed a permeable aortic stent graft wi-thout endoleak (Figure 7).
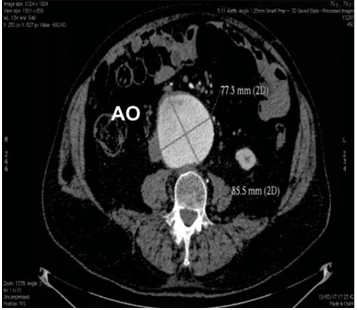
Figure 1. Measurements of the maximum diameters of the aortic aneu-rysm. AngioCT with contrast, axial section.
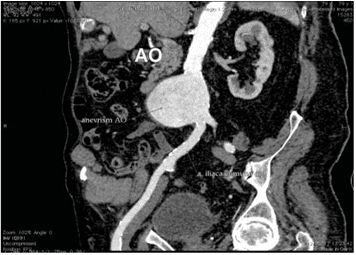
Figure 2. Measurements of the maximum diameters of the aortic aneu-rysm. AngioCT with contrast, sagital section.
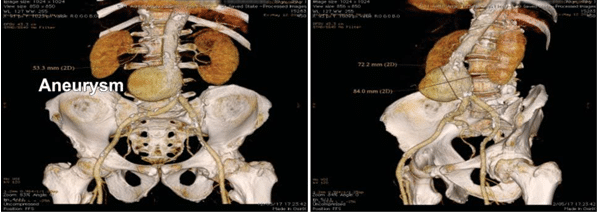
Figure 3. AngioCT reconstruction demonstrated the diameter of aortic aneurysm and the length of aortic neck.
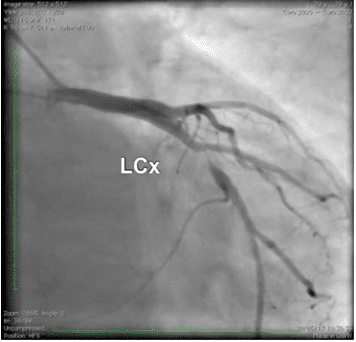
Figure 4. Coronary angiography. Stenosis of left circumfl ex (LCx) ar-tery.
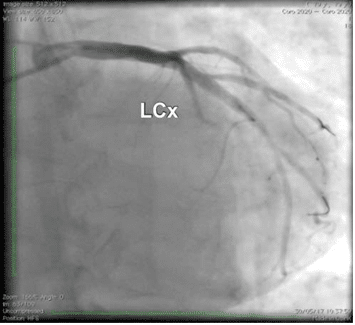
Figure 5. Coronary angiography. Left circumfl ex after stenting.
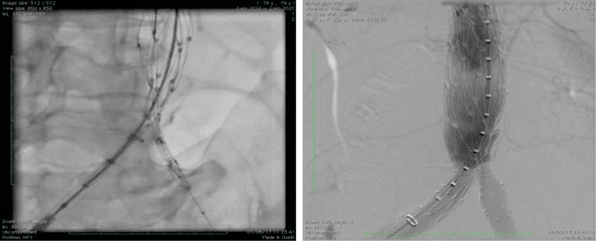
Figure 6. Angiography. Deployment of stentgraft and fi nal result.
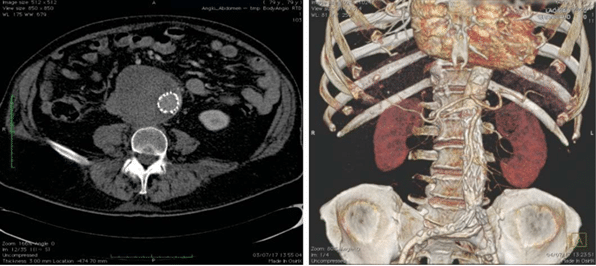
Figure 7. Abdominal angio-CT at 1 month follow up.
DISCUSSION
During the last two decades, endovascular technology revolutionized the management of patients with AAA. Today, EVAR represents the treatment of choice for the majority of patients with an AAA. 2 EVAR has been shown to be associated with reduced periopera-tive morbidity and mortality, as well as with the length of hospitalization.
The first patients undergoing EVAR were treated with tubular grafts that were anchored to the aortic wall by balloon expandable stainless steel stents. At an early stage, aortouniiliac devices combined with femo-ro-femoral bypass and an occluding plug in the contra-lateral common iliac artery were used. EVAR has gai-ned popularity once the appearance of bifurcated and modular stent grafts, which are now used in the vast majority of abdominal EVAR cases. A two- or three-piece modular design permits customization to meet a variety of anatomical and pathological conditions2. Most current systems are based on self-expandable nitinol stents with Dacron or ePTFE fabric. Proximal anchoring hooks reduce the risk of migration, where-as the possible advantage of transrenal fi xation with suprarenal bare metal stent is debated2.
In EVAR successful proximal endograft fi xation and sealing is critical to avoid both migration and endoleak type I3. Several endografts have used different moda-lities to acquire proximal fixation, such as high radi-al force, suprarenal fixation, columnar rigidity, barbs, hooks or anchors4.
The AFX device consists of a main bifurcated uni-body stent-graft system and a proximal aortic exten-sion, which affix firmly to the aorta and provides sea-ling at the aortic neck, while reducing the possibility of stent’s migration at the same time5. AFX2 is a newly designed endograft which offers fixation and sealing of both landing zones4. Thus, it represents the only graft which provides anatomical fi xation to the aortic bifur-cation, since all other grafts use the infrarenal neck as the main fixation point3. The prosthesis migration is prevented because the endograft is deployed since it’s bifurcated component remains at the level of aorto-iliac bifurcation. The skeleton of the device is made of a cobalt-chromium alloy in a multilinked self-expan-ding unibody. External to the stent, the fabric is made of multilayer ePTFE material (STRATA). The stent is attached only to the proximal and distal ends at the proximal aortic extension and allows ePTFE to move independently and conform to abnormal surfaces, faci-litating the sealing of the aneurysmal sack4.
There are a lot of reasons for choosing this type of prosthesis:
-Firstly this stent-graft preserves the aortic bifur-cation for future endovascular procedure and the snare technique allows device to accurate place-ment over aortic bifurcation.
-Secondly, AFX represents the only EVAR system that utilizes a single low profi le 17 F introducer sheath, contralateral access being accomplished percutaneously with a small-diameter sheath (7-French).
-Thirdly, it has a hydrophilic coating for smooth delivery and sheath- dilator system provides exceptional pushability and tracking in tortuous vessels so no gate cannulation is required (the usually obligatory and time-consuming).
-Last but not least, highly conformable ePTFE ma-ximizes wall contact and the device is predictable precise and easy to use. Moreover it prevents the risk of migration as the endograft is anatomi-cally fi xed directly on the aortic bifurcation.
-The initial experience with the AFX stent graft system is promising, with successful aneurysm exclusion and good mid-term results with a low rate of device related complications. Further larger studies are needed to fully evaluate the long-term results4.
CONCLUSION
Upon our best knowledge, this was the second Ro-manian implantation of this new type of aortic endo-prosthesis. The use of a special type of endoprosthe-sis in a patient with a large sized aortic aneurysmus with cumulative anatomic particularities, which could be hardly managed with classical techniques, was safe and overcomes the difficulties related to anatomical characteristics and the need of chronic anticoagulation therapy.
Conflict of interest: none declared.
References
1. Saum AR, Vincent LR, at colab. Abdominal Aortic Aneurysm Clinical Presentation. 2017 aug; Medscape the heart.org
2. Johnny S, Mario L, Frank JV, Anders W. Endovascular Grafts for Ab-dominal Aortic Anurysm. Eur Heart J. 2016; 37 (2): 141-151.
3. Maaz G, Zvonimir K. Endoluminal Abdominal Aortic Aneurysm Re-pair. The latest advances in prevention of distal endograft migration and type 1 endoleak. Tex Heart Inst J. 2010; 37(1): 19-24.
4. George NK, Petroula N, Vasilios B, Michalis P, Aikaterini D, Niko-laos R, Athanasios G, and Miltiadis IM. Initial Clinical Experience with the Endologix AFX Unibody Stent Graft System for Treating Patients with Abdominal Aortic Aneurysms: A Case Controlled Comparative Study. Vascular Specialist International Vol. 33, No. 1, March 2017.
5. Welborn BW, Huey McDB, Johnson CR, Kennedy ER, et al. Clinical outcome of an extended proximal seal zone with AFX endovascular aortic aneurysm system. Journal of Vascular Surgery 2014; Vol. 60, No 4: 876-884.
 This work is licensed under a
This work is licensed under a