Dan Bindea1,2, Bogdan Vasiliu1, Silviu Salomie1, Stanca Aszalos3, Camelia Ober4, Calin Homorodean2,5
1 “Niculae Stăncioiu” Heart Institute, Department of Cardiovascular Surgery, Cluj-Napoca, Romania
2 “Iuliu Haţieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania
3 “Niculae Stăncioiu” Heart Institute, Department of Anaesthesiology, Cluj-Napoca, Romania
4 “Niculae Stăncioiu” Heart Institute, Department of Cardiology, Cluj-Napoca, Romania
5 County Emergency Hospital, Department of Cardiology, Cluj-Napoca, Romania
INTRODUCTION
The ventricular septal rupture is a rare complication (1-2% of all patients with myocardial infarction), with a decreasing incidence (0.2%)1, due to increased early myocardial revascularization possibilities. In the absence of an adequate surgical treatment, mortality is extremely high, being about 25% on the first day, 50% in the first week and 80% in the first month2. This is due to the syndrome of cardiac insufficiency and to quickly installed multiple organs failure.
CASE PRESENTATION
Patient aged 51 years, admitted in our clinic at 5 days after STEMI myocardial infarction with left anterior descending artery (LAD) occlusion without revascularization (Figure 1), for chest pain recurrence and dyspnoea. Electrocardiogram at the time of admission shows pathological Q wave and ST elevation in D1, aVL, V1-V4. Transthoracic echocardiography shows an antero-apical rupture of the ventricular septum with a diameter of 18-20 mm, a left to right shunt, Qp/Qs = 2.5 (Figure 2), akinesia of the anterior wall and of the anterior part of ventricular septum in the 1/3 distal segment, FE = 35%, non-dilated right ventricle. Enzymes of cardiac necrosis are moderately elevated. The patient is hospitalized in Intensive Care Unit (ICU), hemodynamically stable, where an intra-aortic balloon pump (IABP) is introduced. At 24 hours after admission, a deterioration of the liver (ASAT: 82 158 u/l, ALAT: 94 225 u/l) and renal (urea: 55, 71 mg/dl, creatinine: 1.23 – 1.57 mg/dl) functions are noticed, as well as a mild increase of the diameter and of the shunt flow of the ventricular septal
rupture. Under these circumstances, immediate surgery is decided. Cardiopulmonary the bypass is established through a median sternotomy. The access to the ventricular septal rupture is performed through a 6 cm long incision, 1 cm at the left the side of the LAD, in the area of infarction. The ventricular septal rupture is situated antero-apically, with a diameter of about 2 cm (Figure 3). Because of the necrotic area of “soft” infarction, which extends to the ½ lower septum, direct suture with a patch to strictly close the defect is impossible. Given this situation, the “technique of exclusion” is used (described by Tirone David)3: a large patch of bovine pericardium, 7/5 cm, is sutured at distance from the area of rupture and extended from the posterior septum, to its base (to the mitral annulus) and on the outside, towards the left side edge of the ventriculotomy (Figure 4). Thus, the area of rupture is excluded, and the risk of dehiscence subsided, a “new interventricular septum” appears, which will allow the left ventriculotomy to turn into a right ventriculotomy (with
low pressure and reduced risk of bleeding). The postoperative evolution was good, the patient left the ICU department on the 5th day. The follow up echocardiography, shows the presence of two insignificant residual shunts with combined Qp/Qs = 1.3, with no dilatation of the right ventricle (Figure 5). However, raithe patient shows bilateral pleural collections, significant (1000 ml), serum citrine, which requires multiple pleural punctures. The collections gradually decreased during the same hospital admission. The patient is discharged on the 21st day postoperatively, in good clinical condition, without pleural collections, the echocardiography showing the absence of residual shunts. The follow up echocardiography at 1, 6, 12, 18 months shows the absence of residual shunts and a significant improvement of cardiac kinetics. The patient is in excellent clinical condition (NYHA I).
DISCUSSIONS
The postinfarct ventricular septal rupture is a dramatic complication, with an emergency surgical indication for most patients, but with a perioperative mortality that remains extremely high: 23.5% – 54.1%4. The aim of surgery is to close the interventricular communication and the shunt, thus reducing the pulmonary loading and improving the cardiac output and the hemodynamic stability. The right time for surgery is still under debate. The question that arises is whether immediate surgery in patients with a postinfarction interventricular communication provides better results than the “delayed” surgery, knowing the fragility of the necrotic tissue and the diffi culty to perform “good sutures” at that time
and that place. Current guidelines suggest immediate surgery, regardless of the patient’s clinical status, in order to prevent a further hemodynamic deterioration and shunt increase5. However there are studies that conclude that the best strategy in the treatment of the postinfarct ventricular septal rupture remains the “delayed surgery”, delayed by 2-4 weeks, if the hemodynamic status permits. But this conclusion refers to a small group of patients who are hemodynamically stable4,6. The “delayed surgery” strategy gets in contradiction with the existing high percentages of mortality in these patients, during the first month2. Therefore, in most cases, early and aggressive surgery is indicated. In the case of our patient, surgery was imposed after 24 hours of surveillance in the ICU with an IABP, because of the significantly increasing shunt fl ow, the alteration of hepatic and kidney labs. We felt like “delayed
surgery” could have led to an important increase of pulmonary circulation and to the appearance of the hepato-renal failure, which did not justify the time spent up to the cicatrization of myocardial tissue. The percutaneous closure of post infarction ventricular septal rupture with Amplatzer devices can be an option, as a “bridge” to surgery, in patients with cardiogenic shock, in the idea of performing the surgery in a more stable patient7. However, these interventional procedures have shown their limits because of complications
occurring in a large percentage (41%):7-9 major residual shunt, ventricular free wall rupture, device embolisation, as well as a high mortality of 35%10. Recent studies suggest the possibility of using mechanical circulatory assistance (Impella Recover LP) as a bridge towards the surgical closure of the rupture or to transplantation11. This mechanical assistance can be introduced percutaneously, and help decreasing the Qp/Qs and the pulmonary artery and capillary pressure, as well as increasing the cardiac index. Although it has been used for small series of patients, it seems like its benefi ts exceed those of the IABP12. Although the rupture of the ventricular septum had a diameter of only 18-20 mm, it was not possible to directly close it with a circular patch because of the fragility of the septum in the anterior 2/3 and of the impossibility of performing „good sutures” at this level. Under these circumstances, we decided the use of exclusion technique (Tirone David)3 by suturing a large patch of bovine pericardium, redundant, which was attached at distance from the infarction area and stuck to the true ventricular septum by the high pressure in the left ventricle.
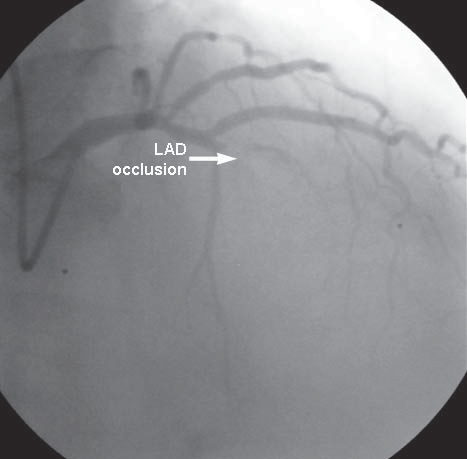
Figure 1. Coronarography (right anterior oblique incidence): LAD occlusion. LAD: left anterior descending artery.
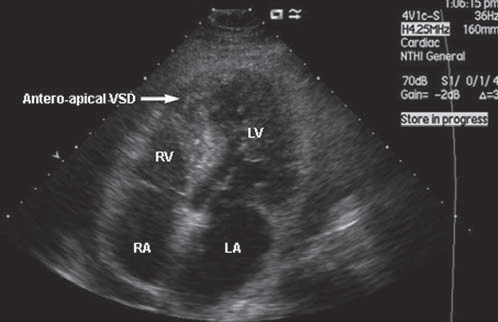
Figure 2. Transthoracic echocardiography (4 chambers incidence) anteroapical VSD. VSD: ventricular septal defect; RA: right atrium; RV: right ventricle; LA: left atrium; LV: left ventricle.
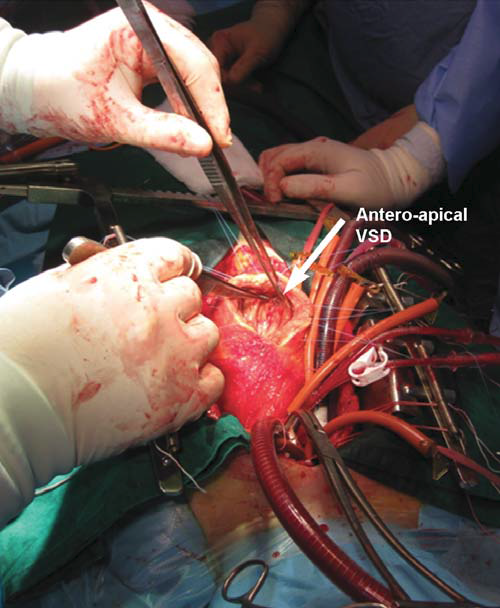
Figure 3. Large left ventriculotomy exposure: antero-apical VSD with infarcted septum. VSD: ventricular septal defect.
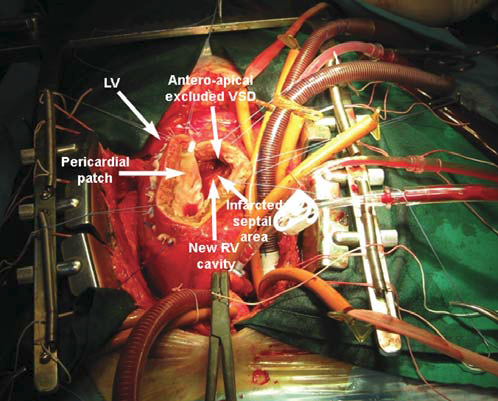
Figure 4. Exclusion technique: large pericardial patch sutured at distance from the VSD. VSD: ventricular septal defect. RV: right ventricle; LV: left ventricle.
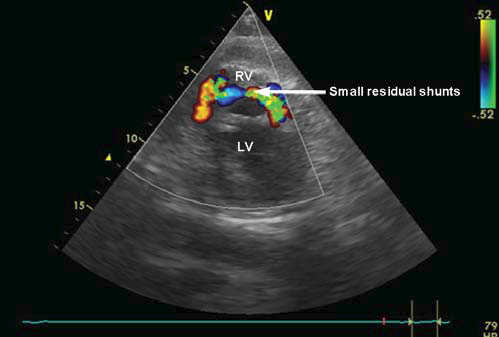
Figure 5. Postoperative transthoracic echocardiography (short axis): 2 small residual shunts. RV: right ventricle; LV: left ventricle.
The patch is just “fi xed” by the anatomical structures described above, causing the
reduction of the shunt with all consequences arising from here. The persistence of the two shunts (the complication is described in literature at about 45% of patients operated for ventricular septal rupture)13 associated with the presence of repetitive pleural collections raithe sed the issue of an aggressive or conservative therapeutic attitude. The residual shunts with hemodynamic impact involve either the iterative surgery with a reinforced patch suture in the dehiscence area, or using the Amplatzer device for closing the residual shunts.
Because the total shunt fl ow was low, with a total Qp/ Qs = 1.3, the patient being hemodynamically stable, without signs of hepato-renal failure, we preferred to adopt a conservative attitude: echocardiography repeated after 3 days, puncture of the pleural collections if necessary (5-7 days) and specifi c medication. Finally, after 3 weeks post-surgery, shunts and pleural collections disappeared and after 18 months the evolution is excellent.
CONCLUSIONS
The surgery of the ventricular septal rupture remains a challenge for the cardiac surgeon, due to high mortality in the absence of surgery and of the high perioperative mortality.
Perioperative mortality decreases signifi cantly if there is an interval of 2-4 weeks before surgery, but in most cases this is impossible because of the failure of the biventricular function, of the increase of the shunt fl ow and the evolution of the hepato-renal insuffi ciency. This is why an aggressive surgical attitude is indicated most of the time. The “exclusion technique” of the inter-ventricular septal rupture, although technically more diffi cult, allows you to suture the patch at distance, thus avoiding the fragility and dehiscence of
infarction areas. Amplatzer devices and modern-type ventricular assistance devices, Impella – type, can provide for selected patients, the time required, as a “bridge” towards a
safer surgery or cardiac transplantation, but this has to be confirmed by future studies.
Conflict of interest: none declared.
References:
1. Crenshaw BS, Granger CB, Birnbaum Y, Pieper KS, Morris DC, Kleiman NS. Risk factors, angiographic patterns, and outcomes in patients with ventricular septal defect complicating acute myocardial infarction. GUSTO-I (Global Utilization of Streptokinase and TPA for Occluded Coronary Arteries) Trial Investigators. Circulation 2000;101:27-32.
2. Agnihotri AK, Madsen JC, Daggett WM. Surgical treatment of complications of acute myocardial infarction: postinfarction ventricular septal defect and free wall rupture. In: Cohn LH (ed.) Cardiac surgery in the adult, New York: McGraw-Hill Medical, 2012: 606.
3. David TE, Armstrong S. Surgical repair of postinfarction ventricular septal defect by infarct exclusion. Semin Thorac Cardiovasc Surg. 1998;10:105-110.
4. Papalexopoulou N, Young CP, Attia RQ. What is the best timing of surgery in patients with post-infarct ventricular rupture? Interact CardioVasc Thorac Surg. 2013;16(2):193-197.
5. Antman EM, Anbe DT, Armstrong PW, Bates ER, Green LA, Hand M. ACC/AHA guidelines for the management of patients with ST-elevation myocardial infarction – executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation 2004;110:588-636.
6. Arnaoutakis GJ, Zhao Y, George TJ, Sciortino CM, McCarthy PM, Conte JV. Surgical repair of ventricular septal defect after myocardial infarction: outcomes from the Society of Thoracic Surgeons National Database. Ann Thorac Surg. 2012;94(2):436-444.
7. Dawson AG, Williams SG, Cole D. Does the placement of an Amplatzer septal occluder device confer benefi t in patients with a post-infarction ventricular septal defect? Interact CardioVasc Thorac Surg.2014;19(6):1040-1047.
8. Hajj-Chahine J, Roth G, Freger N, Vallat A. eComment. Percutaneous closure of post-myocardial infarction septal defect. Interact Cardio-Vasc Thorac Surg. 2013; 16(2):196.
9. Attia R, Blauth C. Which patients might be suitable for a septal occluder device closure of postinfarction ventricular septal rupture rather than immediate surgery? Interact CardioVasc Thorac Surg.2010;11:626-629.
10. Thiele H, Kaulfersch C, Daehnert I, Schoenauer M, Eitel I, Borger M. Immediate primary transcatheter closure of postinfarction ventricular septal defects. Eur Heart J. 2009; 30:81-88.
11. Torre MW, Centofanti P, Attisani M, Patanè F, Rinaldi M. Posterior ventricular septal defect in presence of cardiogenic shock: early implantation of the impella recover LP 5.0 as a bridge to surgery. Tex Heart Inst J. 2011;38:42-49.
12. Seyfarth M, Sibbing D, Bauer I, Fröhlich G, Bott-Flügel L, Byrne R. A randomized clinical trial evaluate the safety and effi cacy of a percutaneous left ventricular assist device versus intra-aortic balloon pumping for treatment of cardiogenic shock caused by myocardial infarction. J Am Coll Cardiol. 2008;4:1584-1588.
13. Zhang B, Liang J, Zheng X, Jiang G, Yang Z, Zhang L, Zhang Y, Sun H. Transcatheter Closure of Postoperative Residual Ventricular Septal Defects Using Amplatzer – Type Perimembranous VSD Occluder. J Invasive Cardiol. 2013; 25(8):402-405.
 This work is licensed under a
This work is licensed under a