Oana Andrei1, Radu Ciudin1, Carmen Ginghina1
1 Institute of Emergency for Cardiovascular Diseases “Prof. Dr. C.C.Iliescu”, Bucharest, Romania.
INTRODUCTION
Along with increasing population age the prevalence of conduction disorders has also increased. The only effective therapy of symptomatic conduction disorders is pacing. Each year ~750000 new pacemakers1 are implanted. Despite the clinical investigations conducted for nearly 20 years, the optimal way of pacing remains a controversial issue. Atrioventricular conduction disturbances2,3, left bundle branch block (LBBB)4,5 and right ventricular pacing6,7 show an impact on mitral regurgitation and ventricular function even in patients with preserved left ventricular ejection fraction (LVEF). The magnitude and mechanics by which they influence mitral regurgitationare still not quantified.
FUNCTIONAL MITRAL REGURGITATION
The mitral valve apparatus is a dynamic, three-dimensional complex, whose normal function depends on the integrity and harmonious interaction of its components. The key element of functional mitral regurgitation is the ventricular-valvular interaction, which appears as a result of the mitral valve dysfunction evolving secondarily to the LV (left ventricle) damage and atrioventricular (AV) dyssynchrony. Functional mitral regurgitation occurs in approximately 30% of patients with ischemic heart disease and dilated cardiomyopathy and brings about a negative prognosis, being an independent predictor of mortality8. Functional mitral regurgitation is caused by the imbalance between tethering forces that tend to pull the mitral valve cusps towards the left ventricle (LV) (LV dilatation, mitral annulus dilatation, papillary muscle displacement, LV sphericity) and closing forces of the mitral valve (decrease in LV contractility, global LV contraction dyssynchrony, papillary muscle dyssynchrony, reduction of systolic contraction of the papillary muscles)7-14. Tethering forces are transmitted through the LV wall–papillary muscles – chordae tendineae system and do not allow the cusps to prolapse into the left atrium (LA). Closing forces depend mostly on the force generated by the LV to close the mitral valve cusps. Thus, in dilated cardiomyopathy, functional mitral regurgitation is caused by increase of tethering forces (geometric remodeling of the LV induces the apical and lateral displacement of the papillary muscles and the tethering, dilatation and fl attening of the mitral annulus) and decrease of closing forces due to systolic dysfunction. Additionally, dyssynchrony of papillary muscles and underlying myocardium can be associated. In the case of a LV with normal size and function (the case of atrioventricular and intraventricular conductions disorders and cardiac pacing), the possible mechanism of functional mitral regurgitation is less known, atrioventricular dyssynchrony can be addressed by the absence of normal temporal relationship of atrial and ventricular phases during the cardiac cycle, intraventricular dyssynchrony with altered activation sequence of the myocardium, papillary muscles and consequently the mitral apparatus, or cusps’ coaptation abnormalities15. Recent data support the idea that the mitral valve is not an „innocent bystander” in patients with chronic functional mitral regurgitation. An adaptive process of structural remodeling occurs in the mitral valve cusps through changes in the extracellular matrix by their thickening and lengthening, proportionally to the tethering of papillary muscles, mitral annulus dilatation, LV and LA dilatation. The area of mitral valve cusps can increase up to 35%, reducing initially the degree of mitral regurgitation. In time, remodeling becomes insuffi cient and contributes to the worsening of the mitral regurgitation. It triggers a vicious circle: mitral regurgitation represents a volume overload of the left ventricle, which remodels and dilates itself in order to restore parietal pressure. Alteration of the LV geometry determines the remodeling of the mitral annulus, cusps and chordae tendineae with aggravation of mitral regurgitation. Thus, the severity of mitral regurgitation increases during these processes of adaptation, being progressive, in constant motion, fueling the LV remodeling in progress8,10,17. The severity of functional mitral regurgitation varies during the cardiac cycle, because it is dependent on the loading conditions and phases of cardiac cycle8,17-19. According to Carpentier’s functional classification, functional mitral regurgitation is of type IIIB/I, being determined by the systolic restriction of mitral valve cusps’ displacement and mitral annulus dilatation8,9. The cornerstone in evaluating functional mitral regurgitation is both the transthoracic and transesophageal echocardiography that play complementary roles. Transesophageal ultrasound identifies more accurately the cause and mechanism, it measures the cusps’ length, the angles (especially the posterolateral angle – meaning the posterior cusp traction)8,9. Ultrasound is indispensable to assess mitral valve anatomy, LV size and function, right ventricle (RV) and LA, pressure in the pulmonary artery, quantification of tricuspid regurgitation that are required for a successful repair of mitral regurgitation. Integration of qualitative, quantitative and semi-quantitative assessments classify it into mild, moderate and severe. 1. Semi-quantitative parameters: vena contracta, flow in the pulmonary veins (peak velocities of systolic (S) and diastolic (D) waves, duration and maximum velocity of the atrial reflux (Ar) wave, S/D ration), IVTMI/IVTTEVS ratio (velocity time integral of mitral valve/velocity time integral in the LV outflow tract)9,12,14; 2. Quantitative parameters: regurgitant orifice area, regurgitant volume9,12,14;
3. Prognostic parameters of functional mitral regurgitation9,12,14: – parameters reflecting the deformation of the
mitral apparatus: coaptation distance, tenting area, posterolateral angle, mitral annulus, mitral annulus contraction;
– parameters that reflect local remodeling: the distance between papillary muscles, delay between contraction of papillary muscles, apical displacement of the posteromedial papillary muscle, – parameters that reflect local remodeling: LV telesystolic diameter, LV telediastolic diameter, LV telesystolic volume, sphericity index.
4. The dilated LA and LV support the diagnosis9,12,14. Echocardiographic quantification of mitral regurgitation
severity is based on the assessment of the central portion of regurgitant flow by measuring the vena contracta or proximal convergence zone. Since these methods admit that the mitral orifice is circular, functional mitral regurgitation is undervalued by their measurement, the mitral orifice in its case being elliptical. This situation is further aggravated if there are multiple jets. 3D echocardiography exceeds this limit by direct planimetry of the vena contracta regardless of the form and number of orifi ces. The severity of mitral regurgitation varies during the cardiac cycle phases, and it can be more important during the proto- or tele-systole. Therefore, its severity is undervalued if done during the meso-systole. The severity of mitral regurgitation is assessed after integrating all echocardiographic parameters obtained by 2D and 3Dmethods8. Exercise echocardiography can bring additional data on the severity of functional mitral regurgitation. This is necessary if there are inconsistencies between clinical data and severity of functional mitral regurgitation evaluated at rest. At effort, functional mitral regurgitation can worsen as a result of hemodynamic changes (increase of LV pre- and afterload) induced by it. The LV may become more spherical, causing the increase of the cusps’ coaptation distance and the systolic expansion of the mitral annulus. Moreover, the effort may induce the increase of LV dyssynchrony with the worsening
of the functional mitral regurgitation. Although there are studies showing that the presence of functional mitral regurgitation at effort identifi es patients at increased risk of mortality, larger trials are necessary to determine the role of exercise echocardiography in its evaluation8. Regardless of etiology, functional mitral regurgitation is associated with increased mortality from any cause and increase of hospitalizations for heart failure (HF)8.
VENTRICULAR-MITRAL-ATRIAL INTERRELATION IN CONDUCTION DISORDERS AND CARDIAC PACING
Atrioventricular blocks of any degree determine diastolic mitral regurgitational contraction (it occurs early in diastole and it is followed by an incomplete closing the mitral valve) and the ventricular systole (that normally determines the efficient closing of the mitral valve) and due to the reversal of the atrioventricular pressure gradient (telediastolic pressure in the LV is higher than the pressure in the LA, atrial contraction being followed by its relaxation thereof). In most cases (when the telediastolic pressure of the LV is not elevated), diastolic mitral regurgitation has a low hemodynamic signifi cance2,3. Most conduction disorders require pacing as directed by current guidelines20. Pacing of patients with preserved systolic function, according to these indications, may be single-chamber or dual-chamber pacing. In both cases, the ventricular electrode is located in the right ventricle (RV), most often in the RV apex, this location being easily accessible, ensuring a safe and effective pacing by using an endocardial electrode with passive fi xation21,22. Apical pacing of RV has adverse hemodynamic consequences, similar to those induced by the left bundle branch block (LBBB), due to non-physiological activation of the LV23. Single-chamber ventricular pacing as treatment of symptomatic AVB are multiple adverse hemodynamic consequences, being determined by the atrioventricular (AV) dyssynchrony (with loss of atrial pump contribution to cardiac output and occurrence of diastolic functional mitral regurgitation), by the ventriculoatrial conduction (underlying the pacemaker syndrome) and by the intraventricular and interventricular dyssynchrony due to RV pacing24,25. Since 1925, Wiggers has showed that RV pacing reduced the LV’s pump function in mammals26,27. Dual-chamber pacing, although preserved the atrioventricular synchrony, involves a compromise between restoring the stimulated, reasonable heart rate and unwanted cardiac dyssynchrony induced by RV pacing28. Approximately 50% of patients with RV pacing develop dyssynchrony in the LV contraction, followed by alteration of the systolic function and by its remodeling29- 32. Hemodynamic benefi ts of atrioventricular synchrony are: 1) increases the preload and, thereby the LV contractility; 2) limits mitral regurgitation by closing the AV valves before ventricular systole; 3) facilitates venous return by maintaining a low pressure in the LA; 4) regulates the autonomic and neurohormonal refl exes involving LA26. Randomized clinical trials have showed that atrial pacing (keeping atrioventricular and intraventricular synchrony) reduces the risk of HF, atrial fibrillation and mortality compared with the dual-chamber or singlechamber ventricular pacing. The target concern is to develop algorithms for decreasing the unnecessary ventricular pacing. The first evidences of this concept come from the MOST trial analysis, which shows that the higher cumulative percentage of RV pacing correlates with the higher risk for HF and atrial fi brillation in patients with SND (sinus node dysfunction). The cumulative percentage of RV pacing above 80% in the VVI mode is the strongest predictor of HF post implantation. The lowest risk have had the patients with a low cumulative percentage of RV pacing (those with atrial pacing in dual-chamber pacemaker context)34. The results of the DAVID and MADITII trials are consistent, reaching the conclusion that the lowest risk of HF worsening and mortality is with patients having a low cumulative percentage of V pacing6,26,33. RV pacing and LBBB induce the heterogeneous electrical and mechanical activation of the myocardium, because the wave front propagation is done slowly, more in the myocardium than in the specialized conduction system, His-Purkinje. Asynchronous electric activation has adverse hemodynamic consequence because not only the onset differs at the level of the ventricular myocardium, but also its contraction pattern. Thus, delayed hemodynamic activation between different myocardial segments occurs with global hemodynamic consequences. The strain of myocardium near the electrod contracts earlier and determines the tethering of the non-activated remote myocardium. This tethering further delays their shortening, increasing he local contraction force (based on the Frank Starling, local mechanism) and is an important stimulus of the remodeling process, causing the more pronounced hypertrophy in the pre-stretched myocardium regions activated later. The delayed activated myocardium tractions through early activated myocardial areas, subjecting them to a paradoxical systolic stretch. This mutual stretch of segments in the LV myocardium causes a less efficient mechanical and energetic systolic contraction and an abnormal relaxation. Asynchronous contraction determines the shortening of the ejection time and slowing down of the decrease and increase rate of LV pressure, causing the lengthening of the periods of isovolumetric contraction and relaxation33 and a reduced stroke volum. Abnormal relaxation is caused by the premature relaxation of the early activated myocardium regions and delayed contraction of remote myocardial segments. This is expressed through the reduction of the LV pressure drop rate, increase in relaxation time, decrease of E-wave velocity. Through the delayed activation and contraction (elongation of the LV systole), V pacing decreases the diastolic fi lling period and the preload. Prolongation of the LV systole causes the occurrence of interventricular dyssynchrony (RV contraction begins before LV contraction)33. During RV pacing, the strain in the different myocardial segments becomes asynchronous with different onset of shortening6,33,35. STE provides an accurate assessment of LV function and mechanical asynchrony. Thus, a decrease of the longitudinal strain in the myocardial segments near the electrode is found (activated earlier) and is increased in the remote myocardial segments6,33. Local differences in myocardial contraction pattern during pacing determines the redistribution of mechanical work, of perfusion and of oxygen required in the ventricular wall. These are mainly in the apical and inferior segments, where electrodes of the V pacing are located, being irreversible after pacing is off. Thus, induction of varying degrees of myocardial injury and interstitial fi brosis in different myocardial segments causes heterogeneous conduction disorders, increasing the likelihood to develop mechanical dyssynchrony. Dystrophic calcifi cations, disorganization of myocardial mitochondria and LV myocardium myofibrils are described in V pacing. Early signs of cellular and molecular changes as a result of abnormal activation of the myocardium are the electrocardiographic repolarization changes occurred after stopping V pacing (T wave morphologic alteration) 33,35. In this phenomenon, Ca and K channels play an important role33. During RV pacing and LBBB, the activation of the LV starts in the interventricular septum (IVS) on the path of slow transseptal conduction. Subsequently, depending on the location of the electrode (more apical or more towards the base of the RV), the specialized conduction tissue (Purkinje fi bers) is engaged in conducting the electric wave front in a higher proportion (as it is positioned more towards the base) or in a lower proportion. Medioseptal pacing or at RV basal IVS level causes a more physiological electrical activation (the concentration of Purkinje fi bers is higher and the QRS complex is narrower) compared with apical stimulation (lower density of Purkinje fi bers)1,6,22,23,31,33,38-48. The duration of ventricular myocardium activation in the presence of RV pacing or LBBB depends on the functional integrity of the specialized distal conduction system. The longer duration of the QRS complex is accompanied by a higher electromechanical delay, correlated with a greater reduction in LVEF and a more severe mitral regurgitation1,6,22,23,31,33,38-49. A study that aimed at measuring some hemodynamic parameters of the LV systolic and diastolic function shows that significant improvement of hemodynamics during RV pacing is obtained by positioning the electrode to the RV free wall towards the septum and from the apex to the base, from the lower to the upper segments. The best mechanical performance of the LV is obtained by pacing the RV septum in the medial superior non-apical segments. This pattern of disorganized electrical activation causes a delay in the depolarization of the inferolateral wall and an asynchronous mechanical contraction42. The anatomy of the mitral apparatus makes the location of the papillary muscles to be close to the myocardial segments activated the earliest (posteromedial papillary muscle) and the latest (anterolateral papillary muscle). Thus, the contraction dyssynchrony induced by the electrical dyssynchrony during RV pacing and LBBB causes the occurrence of mitral regurgitation, initially by delaying the interpapillary contraction and regional remodeling and, thereafter, by global geometry remodeling of the LV6,11,15,17,19,20,25,49-53. As early as 1984, Maurer et al showed that RV pacing induces mitral regurgitation, that its severity is higher in the case of apical location of the stimulation probe and that the mapping of the ventricular myocardium regional function shows a signifi cant heterogeneity, regional contraction disorders are maximum in the vicinity of the stimulation probe54. Understanding the ventricularvalvular interaction helps to develop new therapeutic means. Resynchronization therapy by improving contraction synchrony of papillary muscles, as fi rst mechanism, and subsequently by increasing the contraction strength of the ventricular myocardium and, reversely, its remodeling is a therapeutic option that improves mitral regurgitation8,9, 15,17,19,20,52,53. Since the LA reacts to changes occurred in the LV, RV apex pacing influences the LA function and size. Delay of electromechanical activation caused by RV pacing increases the depolarization time of the LV (LV systole ends after RV systole, inducing interventricular dyssynchrony), so that the early protodiastolic filling of the LV is compromised. Therefore, the residual volume of the LA before atrial systole increases, with increase of its contraction strength, by observing the Frank Starling mechanism at atrial level. In time, overcoming this mechanism is followed by increase of LA pressure and volume, causing the reduction of longitudinal deformation39,55,56. Thus, the atrial elongation during LV systole (LA acts as a reservoir) and proto- and telediastolic atrial shortening (LA acts as a pipe and, respectively, as a pump) are reduced. In addition, functional mitral regurgitation caused by RV pacing contributes to increase the LA volume and pressure. Dilation and increase of atrial pressure amplifies, in its turn, systolic functional mitral regurgitation and favors the occurrence of diastolic mitral regurgitation. Thus, the relationship between the LV and LA function and mitral regurgitation is interactive and dynamic, being crucial to maintain the cardiac output.
CLINICAL IMPLICATIONS
LV systolic dysfunction induced by the RV pacing is determined by the complex interaction between the substrate and promoter34. The substrate is determined by intrinsic atrial rhythm, AV conduction, intraventricular conduction, LV myocardial function (the dysfunction, even if subclinical, both systolic and diastolic, determines additional alteration of electromechanical activation) and the presence of mitral regurgitation and HF34,36. Promoters are specific to pacing and comprise two elements: intraventricular and AV dyssynchrony. Subtle differences in the location of the electrode in the RV apex, depending on the proximity to the Hiss Purkinje system, may influence the appearance of electromechanical V dyssynchrony. Any stimulation outside the normal conduction system finally determines electromechanical changes with the deterioration of the LV function. Patients with substrate at low risk (normal systolic function, normal QRS, without mitral regurgitation, without LVH – left ventricular hypertrophy, and without myocardial infarction) and a low cumulative percentage of RV pacing have a reduced risk of developing HF32,34,36,37. In the MOST study the cumulative percentage of RV pacing over 40% in the DDD mode doubles the relative risk of HF compared with the cumulative percentage of RV pacing below 40%. If the substrate is at low risk, the relative risk increases from 2% to 4%, and if the substrate is at increased risk, the relative risk increases from 20% to 40%, in this case the absolute risk also increases in a statistically significant manner34.The logical approach for selecting the optimal pacemaker is to choose how to prevent and avoid underlying bradycardia and to avoid the cardiac pacing of cavities with normal electrical and conduction properties. The strategies to reduce RV pacing have two approaches. The fi rst approach involves manipulating the pacing manner and period in patients with normal AV conduction, in order to reduce the unnecessary V pacing and to preserve normal intraventricular conduction and it consists of: prolongation of the stimulated AV interval (to allow intrinsic AV conduction), the stimulation rhythm to be under the intrinsic rhythm in the inactivity period (sleep), the stimulation frequency to be the hysteresis frequency, reducing medicines that affect the AV node, programming for adjusting the pacing rate to be reserved for symptomatic patients due to the long AV interval or high-degree AVB. The second approach involves V pacing in alternative places to mitigate the adverse effects of V dyssynchrony.
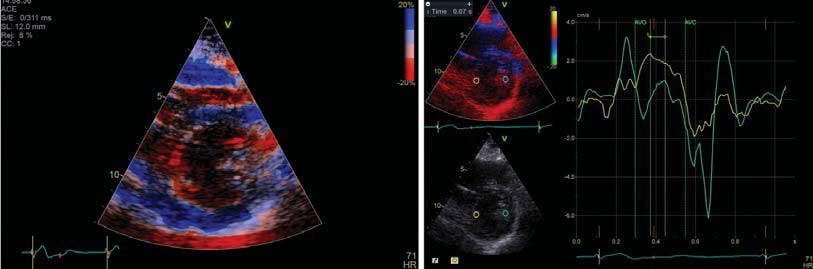
Figure 1. Right ventricular pacing leads to asynchronous contraction and interpapillary contraction delay.
This approach is necessary when the V pacing cannot be avoided due to abnormal AV conduction25,33. Negative hemodynamic effects of RV pacing have given rise to the search for techniques to early identify patients that are vulnerable to develop LV dysfunction following the RV pacing and some strategies to mitigate them. Because echocardiographic imaging by strain can differentiate the active shortening from the passive movement of the parietal myocardium, this is a very useful technique for assessing the effects of mechanical dyssynchrony on the LV function57.Detailed assessment of LV function and mechanical dyssynchrony is achieved by newer, strain echocardiographic techniques through tissue Doppler imaging method (STDI) or speckle tracking (SST). They serve not only for assessing the effects of V pacing on LV mechanics, but also for selection of alternate (more physiological) pacing sites and for selecting vulnerable patients that benefit from this type of pacing or biventricular pacing58. Tissue Doppler method is an important technique in assessing V mechanics and dyssynchrony, but it has some limitations. One of the limitations is that the assessment of myocardial velocities and strain are angle-dependent58. The speckle tracking technique is a more recent echocardiographic technique that allows multidirectional, angle-independent evaluation of the LV mechanics and function. Furthermore, this technique is not infl uenced by the traction effect (tethering) of neighboring myocardial segments, becoming the echocardiographic method to be chosen for assessing temporal differences in myocardial contraction. By using this technique one can assess the mechanical dyssynchrony of the LV (by calculating the differences between the maximum systolic strain moments of different myocardial segments of the LV), LV strain (detects more subtle regional changes compared with the conventional assessment of the LV function by ejection fraction), LV torsion (difference between the rotation of apical and basal segments of the LV, due to the helicoidal disposition of myocardial fi bers). Matsuoka et al have shown that RV pacing causes significant decrease of LV torsion. As a result, fi lling and emptying the LV are less efficient, contributing to the systolic and diastolic dysfunction in patients with V pacing. The global longitudinal strain (GLS) is reported as a better parameter than EF in predicting subclinical LV dysfunction and the prognosis57. LV dyssynchrony induce by RV apical pacing significantly alters the active and passive LA stretching. The results of a study investigating the effect of RV pacing on the LA function divided patients into two groups, depending on the cumulative pacing percentage, showing that, although there are no statistically significant differences in terms of EF, GLS of the LV, the V dyssynchrony parameters, indexed LA index, the strain and the systolic, protodiastolic and telediastolic strain rate of the LA were significantly damaged after 12 months of RV apical pacing. LA function deterioration is significantly correlated with the cumulative pacing percentage55,56. The SST role in choosing alternative stimulation sites is based on the fact that electromechanical activation of the LV is more physiologic by positioning the pacing electrode near the His Purkinje system. The possible sites for RV pacing are: RV outfl ow tract (RVOT), RV septum and directly at the level of the bundle of His. The results of several studies that followed up comparatively, patients with LVEF >50% that were pacemaker- dependent, with pacing in the RV apex and with alternative localization (RV septum or RVOT) RV pacing, showed that, although there were no statistically significant differences regarding LVEF between the two groups, the global longitudinal strain (GLS) was significantly lower in the group of patients with pacing in the RV apex. Moreover, there are statistically significant differences in terms of ventricular dyssynchrony parameters and duration of the QRS complex – significantly higher in the group of patients with apical pacing, and the apical strain is lower. Inefficient asynchronous contraction and low apical strain appear to be associated with altered global LV function in patients with RV apical pacing1,41,45,47. The PACE trial and Solis et al study show that about 50% of patients with RV apical pacing have impaired LV function. Patients with paframeters of ventricular dyssynchrony one month after pacing, have a 5 times higher risk of developing LV systolic dysfunction after 12 months. These results are maintained after 2 years, supporting the progressive nature of adverse LV remodeling29,30. The intra- and interventricular dyssynchrony triggers a vicious circle, in which structural and histopathological lesions are self-maintained, leading to a loss of contractile function in the early activated myocardial segments and to hypertrophy in the delayed activated myocardial segments, to the occurrence of mitral regurgitation, at the beginning by the absence of temporal coordination of papillary muscles and by regional remodeling, and later by global LV remodeling. Mitral regurgitation cause unfavorable, additional remodeling of the LV. Biventricular pacing reduces the functional mitral regurgitation induced by the electromechanical dyssynchrony through its dual benefi cial effect on the LV contraction and remodeling, improving the closing and tethering forces6-9,15,18,19,25,58 causing:
1) optimization of the V interval with reduction of the contraction period, prolongation of the diastolic filling and reduction of diastolic functional mitral regurgitation;
2) restoration of the interpapillary tem porary coordination by improving the synchrony of
myocardial segments adjacent to papillary muscles;
3)reverse remodeling of the LV by restoring its normal geometry reduces mitral regurgitation by changing the
traction forces (LV volumes decrease, it reduces the mitral annulus area, the closure area of the cusps and
covering volumes (tenting); 4) increase of closure forces of the mitral valve by increasing the LVEF25. Thus,
mitral regurgitation is an important factor that influences the ultimate benefit of CRT.
CONCLUSIONS
At this moment, the impact of AV, interventricular and intraventricular dyssynchrony on mitral regurgitation is
not yet quantified. Using echocardiographic techniques as screening methods for early detection after RV pacing implantation, the occurrence of LV mechanical dyssynchrony and functional mitral regurgitation play an important role. Thus, asymptomatic patients with preserved systolic function might be identified, who could benefit from RV pacing with alternative localization (IVS or RVOT) or biventricular pacing as treatment of bradycardia from the very beginning. Further trials are needed, which track a larger number of patients over a longer period of time to determine the optimal pacing approach. An ongoing study that aims to respond to this problem is ENHANCE61.
Acknowledgement: This paper is supported by the Sectoral Operational Programme Human Resources Development (SOP HRD), fi nanced from the European Social Fund and by the Romanian Government under the contract number POSDRU/187/1.5/S/156040/.
Conflict of interest: none declared.
References
1. G.C.Kaye, N.J.Linker, et al Effect of right ventricular pacing lead site on left ventricular function in patients with high-grade atrioventricular block: results of the Protect-Pace study. Eur Heart J 2015:36:856-862.
2. Agmon Y, Freeman W.K., et al. Diastolic mitral regurgitation. Circulation 1999;99:e13.
3. Schnittger I., Appleton C.P., et al. Diastolic mitral and tricuspid regurgitation by Doppler echocardiography in patients with atrioventricular block: new insight into the mechanism of atrioventricular valve closure. J am coll cardiol 1988;11:83-8.
4. Breithard G., Breithard O.A.. Left bundle branch block, an old-new entity. J of Cardiovasc Trans Res 2012;5:107-16.
5. Erlebacher J.A., Barbarash S..Intraventricular contraction delay and functional mitral regurgitation. The American Journal of Cardiology 2001;88:83-86.
6. Tops L.F., Schalij M.J., et al. The effects of right ventricular apical pacing on ventricular function and dyssynchrony. J AM Coll Cardiol 2009;54:764-76.
7. Dal-Bianco J.P., Levine R.A. Anatomy of the Mitral Valve Apparatus – Role 2D and 3D Echcardiography. Cardiol clin. 2013;31:1-23.
8. Asgar A W., Mack M J., Stone G. W., Secondary Mitral Regurgitation in Heart Failure Pathophysiology, Prognosis, and Therapeutic Considerations. J Am Coll Cardiol 2015:65:1231-1248.
9. Lancellotti P., Moura L., et al. European association of echocardiography recommendations for the assesment of valvular regurgiation. Part 2: mitral and tricuspid regurgitation (native valve disease). Eur J Echocardiogr 2010;11:307-332.
10. Chaput M., Handschumacher M.D et al. Mitral leaflet adaptation to ventricular remodeling Occurrence and adequacy in patients with functional mitral regurgitation. Circulation 2008;118:845-852.
11. Rita M., Sofi a A., et al. Acute severe mitral regurgitation as an early complication of pacemaker implantion. Europace 2010;12:1791-2.
12. Ginghina C., Popescu B.A., Jurcut R.. Esentialul in ecocardiografi e. Ed.Medicala Antaeus 2013;143-164.
13. Marwick T.H., Lancellotti P., et al. Ischemic mitral regurgitation: mechanism and diagnoses. Heart 2009;95:1711-18.
14. Calin A., Ginghina C.. Regurgitarea mitrala In Ginghina C. Mic tratat de cardiologie Ed. Academiei Romane, Bucuresti 2010;451-468.
15. Acosta H., Viafara L.M. et al. Reduction of mitral regurgitation by biventricular pacing with intraventricular timing optimization in patients without a standard indication: A potentian new cardiac resynchronization
Therapy. The Journal of innovations in Cardiac Rhythm Management 2012;3:784-790.
16. Debonnaire P., Amri I Al, et al. Leafl et remodeling in functional mitral valve regurgitation: characteristic, determinants, and relation to regurgitation severty. Eur Heart J – cardiovasculat imaging 2015;16:290-299.
17. Ypenburg C, Lancellotti P. et al. Acute effects of initiantion and withdrawal of cardiac resynchronization therapy on papillary muscle dyssynchrony and mitral regurgitation. J am Coll Cardiol 2007;50:2071-2077.
18. Solis J., McCarty D et al. Mechanism of decrease in mitral regurgitation after cardiac resynchronization therapy optimization of forcebalancerelationship. Circ Cadiovasc imaging 2009;2:444-450.
19. Eskesen K., Kanagalingam S., Abraham T.P. Mitral regurgitation in cardiac resynchronization solving another piece of the puzzle. Circ cardiovasc imaging 2009;2:427-428.
20. Brignole M., Auricchio A., et al. 2013 ESC guidelines on cardiac pacing and cardiac resynchronization therapy. Eur Heart J 2013;34:2281- 329.
21. Furman S., Schwedel J.B., et al. An intracardiac pacemaker for Stokes
–Adams seizures. N Engl J Med 1959;40:232-6.
22. Cock C.C., Giudici M.C., TwiskJ. W.. Comparison of the hemodynamic effects of right ventricular outfl ow-tract pacing with righ ventricular apex pacing. Europace 2003;5:275-278.
23. Vassallo J.A., Cassidy D.M. et al. Left ventricular endocardial activation during Right ventricular pacing: Effect of underlying heart disease. J am Coll Cardiol 1986;7:1228-1233.
24. Cannan C.R., Higano S.T., Holmes D.R. Jr. Pacemaker induced mitral regurgitation: an alternative form of pacemaker syndrome. Pace 1997;20:735-738.
25. Stambler B.S., Varma N. Hemodynamics of cardiac pacing and pacing mod selection in Ellenbogen K.A., Kaszala K. Cardiac pacing and ICDs 6th Edition, Wiley Blackwell, 2014:82-133.
26. Wiggers CJ . The muscular reactions of the mammalian ventricles to artificial surface stimuli. Am J Physiol 1925;37:275-282.
27. Tse H. F., Lau C. P. Selection of permanent ventricular pacing site. J AM Coll Cardiol 2006;48:1649-51.
28. Stockburger M., Boveda S. et al. Long-term clinical effects of ventricular pacing reduction with a changeover mode to minimize ventricular pacing in a general pacemaker population. Eur Heart J 2015;36:151- 157.
29. Yat-Sun Chan J., Fang F., et al. Biventricular pacing is superior to right ventricular pacing in bradycardia patients with preserved systolic function:2-year results of the Pace trial. Eur Heart J 2011;32:2533- 2540.
30. Fang F., Zhang Q. et al. Early pacing-induced systolic dyssynchrony is a strong predictor of left ventricular adverse remodeling: Analysis from the Pacing to Avoid Cardiac Enlargement (PACE) trial. International J cardio 2013;168:723-728.
31. Tops L.F., Suffoletto M.S. et al Speckle-tracking radial strain reveals left ventricular dyssynchrony in patients with permanent right ventricular pacing. J am coll cardiol 2007;50:1180-1188.
32. Fang F., Yat-Sun Chan J. et al. Prevalence and determinants of left ventricular systolic dyssynchrony in patients with normal ejection fraction received right ventricular apical pacing: a real-time three-dimensional echocardiographic study. Eur J Echocardiogr 2010;11:109-118.
33. Sweeney M.O., Prinzen F.W. A new paradigm for physiologic ventricular pacing. J am coll cardiol 2006;47:282-288.
34. Sweeny M.O., Hellkamp A.S. Heart failure during cardiac pacing. Circulation 2006;113:2082-2088.
35. Nahlawi M., Waligora M. et al. Left ventricular function after right ventricular pacing. J am coll cardiol 2004;44:1883-1888.
36. Fang F., Zhang Q. et al. Deleterious effect of right ventricular apical pacing on left ventricular diastolic function and the impct of pre-existing diastolic disease. Eur Heart J 2011;32:1891-1899.
37. Zhang X., Chen H. et al. New-Onset Heart Failure After Permanent Right Ventricular Apical Pacing in Patients with acquired High-Grade Atrioventricular Block and Normal Left Ventricular Function. J Cardiovasc Electrophysiol 2008;19:36-141.
38. Alhous M.H., Small G.R. et al. Right ventricular septal pacing as alternative for failed left ventricular lead implantation in cardiac resynchronization therapy candidates. Europace 2015;17:94-100;
39. Pastore G., Aggio S. et al Hisian area and right ventricular apical pacing differently affect left atrial function:an intra-patients evaluation. Europace 2014;16:1033-1039.
40. Chen Ju-Yi, Tsai W. et al Long-term effect of septal or apical pacing on left and right ventricular function after permanent pacemaker implantation. Echocardiography 2013;30:812-819.
41. Saito M., Kaye G. et al. Dyssynchrony, contraction effi ciency and regional function with apical and non-apical RV pacing. Heart 2015;101: 600-608.
42. Vancura V., Wichterle D. et al. Assesment of optimal right ventricular pacing site using invasive measurement of left ventricular systolic and diastolic function. Euroopace 2013;15:1482-1490.
43. Hillock R., Mond H.G. Pacing the right ventricular outfl ow tract septum:time to embrace the future. Europace 2012;14:28-35.
44. Kaye G., Stambler B.S, Yee R. Search for the optimal right ventricular pacing site:design and implementation of three randomized multicerter clinical trial. Pace 2009;32:426-433.
45. Inoue K., Okayama H. et al. Right ventricular pacing septum preserved global left ventricular longitudinal function in comparison with apical pacing. Circ J 2011;75:1609-1615.
46. Gong S., Su Y. et al. Is outfl ow right ventricular tract pacing superior to right ventricular apex pacing in patients with normal cardiac function? Clin cardiol 2009;32:695-699.
47. Alhous M.H.A., Small G.R. et al. Impact of temporary right ventricular pacing from different site on echocardiographyc indices of cardiac function. Europace 2011;13:1738-1746.
48. Shimony A., Eisenberg M.J. et al. Benefi cial effect of right ventricular non-apical vs apical pacing:a systematic review and meta-anlysis of randomized controlled trial. Europace 2012;14:81-91.
49. Liang Y., Zhang Q. ey al. Incremental value of global systolic dyssynchrony in determining the occurrence of functional mitral regurgitation in patients with left ventricular systolic dysfunction. Eur Heart J 2013;34:767-774.
50. Sassone B., De simone N et al. Pacemaker-induced mitral regurgitation: prominent role of abnormal ventricular activation sequence altered atrioventricular synchrony. Ital heart J 2001;2:441-448.
51. Alizadeh A., Sanati H.R., et al. Induction and aggravation of atrioventricular valve regurgitation in the course of chronic right ventricular apical pacing. Europace 2011;13:1587-90.
52. Kanzaki H., Bazaz R., et al. A mechanism for immmediat reduction in mitral regurgitation after cardiac resynchronization therapy. J Am Coll Cardiol 2004;44:1619-25.
53. Ypenburg C., Lancellotti P., et al. Mechanism improvement in mitral regurgitation after cardiac resynchronization therapy. Eur Heart J 2008;29;757-765.
54. Maurer G., Torres M.A.R., et al. Two-dimnsional echocardiography contrast assessment of pacing-induced mitral regurgitation: relation to altered regional left ventriculat function. J am coll cardiol 1984;3: 986-991.
55. Man Seon-Kim, Cho K. et al. Left atrial responses to acute right ventricular apical pacing in patients with sick sinus syndrome. Echocardiography 2013;30:1042-1048.
56. Choi B., Cho K. et al. Impact of right ventricular apical pacing and its frequency on left atrial function. J cardiovasc Ultrasaund 2012;20:42- 48.
57. Tops L.F., Delgado V. et al. The role of Speckle tracking strain imaging in cardiac pacing. Echocardiography 2009;26:315-323.
58. Sideris S., Poulidakis E. et al. Upgrading pacemaker to cardiac resynchronization therapy: an option for patients with chronic right ventricular pacing and heart failure. Hellenic J cardiol 2014;55:17-23.
59. Tanaka H., Hara H. et al. Comparative mechanical activation mapping of RV pacing to LBBB by 2D and 3D speckle tracking and association with response to resynchronization therapy. J am Coll Cardiol img 2010;3:461-471.
60. Frohlich G., Steffel J. et al. Upgrading to resynchronization therapy after chronic right ventricular pacing improves left ventricular remodeling. Eur Heart J 2010;31:1477-1485.
61. Fang F., Jie Z. et al. Cardiac Resynchronisation Therapy and Heart Failure: Persepctive from 5P Medicine. Cardiac Failure Review, 2015;1:1- 3.
 This work is licensed under a
This work is licensed under a