Carmen C. Beladan1,2, Alexandra G. Smărăndoiu1, Simona Botezatu2, Andreea Călin1, Monica Roşca1, Ioan. M. Coman1,2, Anca D. Mateescu1, Bogdan. A. Popescu1,2, Carmen Ginghină1,2
1 “Carol Davila” University of Medicine and Pharmacy, Bucharest, Romania
2 Institute of Cardiovascular Diseases “Prof. Dr. C. C. Iliescu”, Bucharest, Romania
INTRODUCTION
Recent studies report a decrease in acute MI (MI) mortality in parallel with greater use of reperfusion therapy, primary percutaneous coronary intervention, modern antithrombotic therapy, as well as secondary prevention treatments. Still, mortality rate remains significant reaching 12% at 6 months after acute MI, which justifies the ongoing effort to improve quality of care for these patients1. Thus, identifying prognostic factors for a better risk stratification remains an utmost important goal in this setting. Many previous studies have focused on the risk factors for adverse clinical outcome after acute MI. Age, history of prior MI, cardiovascular risk factors, cardiac biomarkers, Killip class, ejection fraction, infarct location, restrictive left ventricular (LV) filling pattern, left atrial volume, multivessel disease, renal dysfunction are among the parameters correlated with early and late mortality in this setting2. Anterior location of MI has been identified by previous studies as a predictor of poor prognosis. Several studies have compared the clinical outcome of patients after anterior or inferior MI and even after adjusting for infarct size a higher incidence of congestive heart failure and cumulative cardiac mortality was observed in patients with anterior MI3-6. Anterior MI may lead to LV apical dysfunction in a significant proportion of cases. Since apical rotation is the main determinant of torsion and, during diastole, apical recoil contributes to suction and ensures adequate LV fi lling at low pressure7, we hypothesized that the worse outcome in patients with anterior MI may be, at least partly, a consequence of LV apical involvement.
METHODS
We analyzed retrospectively from our database 245 echo cardiographic studies of patients with documented history of LV MI (older than 1 year) referred for echo cardio graphy to EUROECOLAB between January 2014 and May 2015. Patients with either apical or basal akinetic segments, (between 2 and 5 segments) were included in our study and two groups were formed on the basis of the wall motion abnormality location: Group 1 – apical, and Group 2 – basal wall motion abnormalities. All echocardiographic studies were reviewed by a single observer blinded to clinical data and wall motion score index (WMSI) as well as parameters of systolic and diastolic function were assessed on stored images retrieved from the database. Clinical files of the included patients were reviewed for demographic data, cardiovascular risk factors, NYHA class, medication, age of MI. Coronary angiography performed during the hospitalization for MI was available in all patients. The most recent coronary angiography was considered in order to assess the extension of coronary artery disease in each patient. Exclusion criteria were: severe valvular disorders, prosthetic valves, permanent atrial fibrillation and left bundle branch block. For statistical analysis we used the SPSS software v. 19. Our results are expressed as mean±standard deviation or, where indicated, as absolute values or percentage. Variables were compared using T-test, ANOVA and Chi-squared test as appropriate. Statistical significance was fixed at a p value less or equal than 0.05. The contribution of multiple factors to a result was assessed by multivariate analyses.
RESULTS
The final study population included 60 patients (60±9 yr) divided in two groups as previously mentioned.
Table 1. Clinical characteristics of the study groups
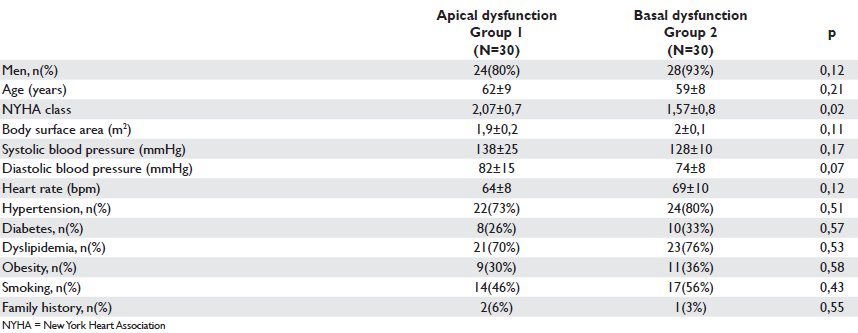
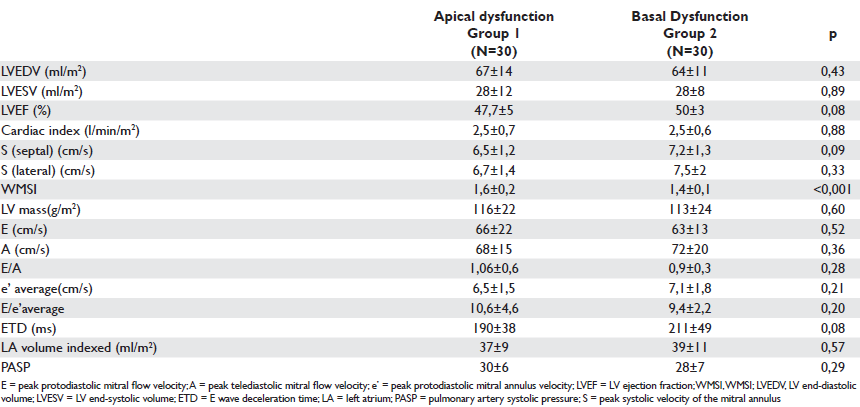
Table 2. Echocardiographic parameters of systolic and diastolic function in the studied groups
Clinical characteristics of the two groups of patients are presented in Table 1. There were no statistically significant differences between groups regarding age, gender, body surface area and values of systolic and diastolic blood pressures or cardiovascular risk factors (p regard to NYHA class, patients with anterior wall motion abnormalities (Group 1) presenting a higher NYHA class as compared to patients with basal wall motion abnormalities (Group 2) ( 2,07±0,7 vs 1,57±0,8; p=0.02). The extension of coronary artery disease according to the most recent coronary angiography available in each patient showed no statistically significant differences between groups. Moreover, no significant differences between groups were observed regarding renal function, medication or age of MI.
Table 3. Clinical characteristics in the two subgroups of patients with similar wall motion score index
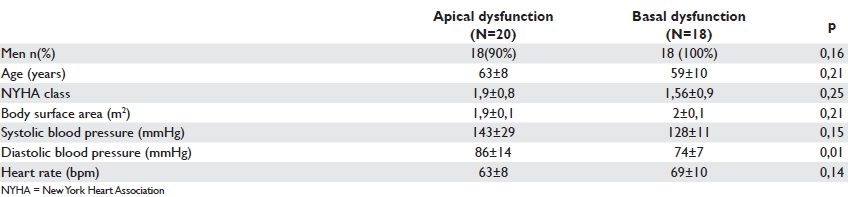
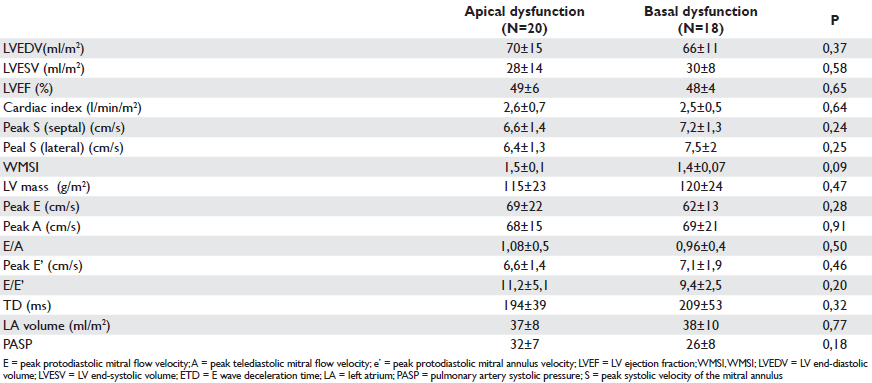
Table 4. Echocardiographic variables in the two groups of patients with comparable WMSI (WMSI)
Echocardiographic parameters of systolic and diastolic function
No statistically significant differences were found be tween the two groups with regard to LV ejection fraction and echocardiographically estimated cardiac index. Still, WMSI was signifi cantly higher in patients with apical dysfunction (1,6±0,2 vs 1,4±0,1; p<0.001). The echocardiographic parameters of LV diastolic function were similar between groups (Table 2).
Correlates of NYHA class in the studied groups
When evaluating the impact of clinical and echocardiographic parameters on NYHA class within the whole study population, we found a significant correlation between the NYHA class index and: LV ejection fraction (p=0.02), WMSI (p=0.02), LV mass index (p=0.03) and diastolic parameters: peak E (p=0.01), E/A ratio (p=0.001), and E/E’ ratio (p<0,001). With multivariate analysis the WMSI remained the only predictor of functional NYHA class. In order to correct for the infl uence of WMSI study on functional status we matched participants according to WMSI and two subgroups were formed: a subgroup of 20 patients with apical wall motion abnormality and a subgroup of 18 patients with basal wall motion abnormality (Table 3). There was no further statistical difference observed between the two groups with similar WMSI and similar echocardiographic parameters of systolic and diastolic function with regard to NYHA class (p=0.25) despite the different location of wall motion abnormalities (Table 4). The main correlates of NYHA class within this group were: peak E (p=0,008), E/A ratio (p=0,001), E/E’ ratio (p=0,00). Multivariate analysis retains E/E’ ratio (p=0.01) as predictor of NYHA functional class.
DISCUSSIONS
The main results of our study are: (1) A poorer functional status in patients with apical vs basal wall motion abnormalities despite similar LVEF and cardiac index was associated in our study with a higher WMSI and thus to a more extensive LV wall abnormality, rather than with actual location of the MI. (2) LV apical dysfunction was not associated in our study with worse echocardiographic parameters of LV diastolic function in Group 1 as compared to Group 2. (3) WMSI emerged as a predictor of NYHA class in the whole group of post-MI patients. (4) No further difference with regard to NYHA class index was found between groups after matching for both WMSI and LVEF irrespective of the location of wall motion abnormalities. E/E’ ratio emerged as a predictor of functional class within this population. Previous studies have identified the anterior location of MI as an indicator of poor prognosis3-6. A study led by Hands et al, which included 398 patients with anterior MI and 391 patients with inferior MI, concluded that anterior location is an indicator of poor prognosis, in both the short and the long term, independently of the size of infarction as evaluated by the levels of peak creatine kinase measured in the acute phase5. The authors mentioned as possible explanations for this finding the qualitative differences between the anterior and inferior walls, the higher risk of dilatation and thinning of the infarcted zone associated with anterior location, as well as the limitations of assessing the extent of damage to the left ventricle via the level of peak creatine kinase considering that patients with an inferior MI can also associate a right ventricular lesion which could have contributed to the rise in creatine kinase. Stone et al obtained similar results after matching patients for size of infarction and found an increased mortality, a higher incidente of congestive heart failure and a lower ejection fraction among patients with an anterior MI4. More recent studies did not provide further insights on this topic. Anterior location of MI may be associated with LV apical dysfunction. Previous studies reported LV apical rotation as the main determinant of systolic torsion whereas apical relaxation during diastole is responsible for a rapid decrease in intraventricular pressure gradient, thus contributing to suction and ensuring the ventricular filling at low pressures7. A study conducted by Opdahl et al underlined the major contribution of apical rotation to the systolic torsion of the left ventricle and also proposed apical rotation, as evaluated by speckle tracking echocardiography, as an index of ventricular torsion8. Data regarding the clinical impact of LV apical dysfunction in patients with anterior MI is scarce. Diminished LV torsion and prolonged untwisting was reported by Takeuchi et al in 30 patients with anterior MI and abnormal LV systolic function as a result of reduced apical rotation9. Our study revealed a statistically signifi cant difference regarding the functional status expressed as NYHA class index between the two main study groups. Thus, a higher NYHA class index was found in patients with apical wall motion abnormality compared to the patients with basal wall motion abnormality despite similar LVEF and cardiac index. There was no statistical difference between groups regarding echocardiographic parameters of diastolic function although higher values for the E/A ratio, shorter deceleration time of E wave velocity and higher average E/E’ ratio were found in the apical dysfunction group. Among the echocardiographic parameters of systolic function a statistically significant difference between groups was noted with regard to the WMSI despite similar LVEF and ecocardiographically assessed cardiac index. Thus, patients with anterior MI presented a more extensive wall motion abnormality. Moreover the WMSI was independent predictor of NYHA class index in the whole study population. This tendency of anterior MI to be more extensive than inferior MI was already described by Thanavaro et al in a study that demonstrated the independent prognostic value of anterior infarction location after correction for infarction size assessed by the level of cardiac enzymes10. In our study, after correction for WMSI there was no statistically significant difference between patients with apical versus basal wall motion abnormalities with regard to NYHA class index or echocardiographic parameters of LV performance.
LIMITATIONS
Despite the large number of echocardiographic studies that have been retrospectively evaluated to test our hypothesis, the number of cases included in the present analyses was restricted due to the inclusion criteria requiring a specific location and extension of wall motion abnormalities. Thus, further confirmation of our results in a larger number of patients would be required in order to increase statistical accuracy and draw conclusions over the whole population with MI.
Clinical implications
The prognostic significance of ischemic LV apical involvement in patients with MI have never been studied. However, since the haemodynamic importance of normal LV apical function with regard to an efficient LV performance is well known it would be of interest to retest our hypothesis in a prospective study including a larger population in an attempt of improving the clinical outcome of post MI patients by more refi ned risk stratification.
CONCLUSIONS
In our study the poorer functional status of post-MI patients with apical wall motion abnormality as compared to patients with basal ischemic involvement was rather related to a more extensive ischemic area than to the actual location of akinetic segments and to apical dysfunction. Moreover, WMSI was the main predictor of functional class in our study population.
Acknowledgements: This study was supported by the Sectorial Operational Programme Human Resources
Development (SOPHRD) financed by the European Social Fund and the Romanian Government under the contract number POSDRU/159/1.5/S/137390.
References
1. Task Force on the management of ST-segment elevation acute MI of the European Society of Cardiology (ESC), Steg PG, James SK et al. ESC Guidelines for the management of acute MI in patients presenting with ST-segment elevation. Eur Heart J 2012; 33 : 2569-619.
2. Park HW, Yoon CH, Kang SH, Choi DJ, Kim HS, Cho MC, Kim YJ, Chae SC, Yoon JH, Gwon HC, Ahn YK, Jeong MH; KAMIR/KorMI Registry Early- and late-term clinical outcome and their predictors in patients with ST-segment elevation MI and non-ST-segment elevation MI. Int J Cardiol 2013; 169:254-61
3. Lee KL, Woodlief LH, Topol EJ, Weaver WD, Betriu A, et al. Predictors of 30-day mortality in the era of reperfusion for acute MI. Results from an international trial of 41021 patients. GUSTO-I Investigators. Circulation. 1995;91:1659-68.
4. Stone PH, Raabe DS, Jaffe AS, Gustafson N, Muller JE et al. Prognostic significance of location and type of MI: independent adverse outcome associated with anterior location. J Am Coll Cardiol 1988; 11:453-63.
5. Hands ME, Lloyd BL, Robinson JS, de Klerk N, Thompson PL. Prognostic significance of electrocardiographic site of infarction after correction for enzymatic size of infarction. Circulation 1986;73:885-91.
6. Behar S, Rabinowitz B, Zion M, Reicher-Reiss H, Kaplinsky E, et al. Immediate and long-term prognostic significance of a first anterior versus first inferior wall Q-wave acute MI. Secondary Prevention Reinfarction
Israeli Nifedipine Trial (SPRINT) Study Group. Am J Cardiol 1993;72:1366-70.
7. Popescu BA, Beladan CC, Calin A, Muraru D, Deleanu D, Rosca M, Ginghina C. LV remodelling and torsional dynamics in dilated cardiomyopathy: reversed apical rotation as a marker of disease severity. Eur J Heart Fail 2009;11:945-51.
8. Opdahl A, Helle-Valle T, Remme EW, Vartdal T, Pettersen E, et al. Apical rotation by speckle tracking echocardiography: a simplified bedside index of LV twist. J Am Soc Echocardiogr 2008;21:1121-8.
9. Takeuchi M, Nishikage T, Nakai H, Kokumai M, Otani S, Lang RM. The assessment of LV twist in anterior wall MI using two-dimensional speckle tracking imaging. J Am Soc Echocardiogr 2007;20:36-44.
10. Thanavaro S, Kleiger RE, Province MA, Hubert JW, Miller JP, et al. Effect of infarct location on the in-hospital prognosis of patients with fi rst transmural MI. Circulation 1982;66:742-7.
 This work is licensed under a
This work is licensed under a