Satoshi Nakatani1
1 First published in Heart [Heart 2014;100:1661–1666.] and reproduced with permission. Copyright restrictions apply.
MYOCARDIAL STRAIN
Echocardiographic measures of myocardial strain have emerged as a promising tool to assess cardiac function and predict prognosis in several cardiac conditions (1-3). LVEF currently is the most popular parameter to describe left ventricular systolic function and to predict outcomes in patients with myocardial infarction and heart failure. However, there are some technical limitations to EF such as difficulty of endocardial border tracing and assumptions in the geometry of the LV. Additionally, compared with EF, global longitudinal strain (GLS) derived from 2D speckle-tracking echocardiography appears to be a more reliable functional parameter and provides more useful prognostic data in patients with heart failure (13).
Previous studies have mostly focused on patients in sinus rhythm. However, there are many patients with atrial fibrillation and heart failure (4), especially in those with heart failure with preserved EF. Atrial fibrillation worsens the outcome of heart failure and viceversa (5). Therefore, the assessment of cardiac function in patients with atrial fibrillation also is important6. In a series of 196 patients with atrial fibrillation, Su et al (7) used the ‘index beat’ method to eliminate the issue of varying R-R intervals (8,9). The index beat is taken as the beat following two nearly equal preceding cardiac cycles. The two intervals preceding the index beat must be at least 500 ms duration and the difference between these two beats must be less than 60 ms. Su et al showed that GLS was better than left ventricular EF and systolic mitral annular velocity for predicting cardiovascular events. Patients with a GLS of −12.5% or less had a better cardiovascular event-free survival than in those with a GLS >–12.5%. Assessment of cardiac function in patients with atrial fibrillation oft en is based on the average of measurements from multiple beats, but this approach is time consuming and cum- bersome10. The ‘index beat’ method is an alternate solution in patients with atrial fibrillation that may be more widely applied if validated in other studies.
In addition to assessment of global function, myocardial strain can be used to assess regional myocardial function. Unlike myocardial velocity measured by tissue Doppler echocardiography, myocardial strain is independent of tethering and translational motion. Thus, regional strain measures may be clinically useful and there have been numerous publications attempting to evaluate regional function in patients with various cardiac diseases such as ischaemic heart disease, valvular heart disease and cardiomyopathy (11). Cardiac amyloidosis is a progressive infiltrative cardiomyopathy with a poor prognosis. Its diagnosis is sometimes challenging because a thickened ventricular wall with prominent diastolic and later systolic dysfunction, as assessed with echocardiography, can be due to other prevalent pathologies (12). In fact, a recent study suggests the prevalence of cardiac amyloidosis may be underestimated and is sometimes overlooked in the clinical setting (13). Phelan et al (14) found a unique feature of myocardial strain distribution in cardiac amyloidosis. They studied 55 consecutive patients with cardiac amyloidosis, 15 patients with hypertrophic cardiomyopathy and 15 patients with aortic stenosis (AS). In addition to longitudinal strain, relative apical longitudinal strain was calculated as the ratio of apical longitudinal strain to the average of basal and mid-longitudinal strain. These authors consistently found an ‘apical sparing’ pattern of longitudinal strain in patients with cardiac amyloidosis and a higher relative apical longitudinal strain (>1.0), indicating a larger apical strain value compared with basal and mid-ventricular strain values (Figure 1). By multivariable logistic regression analysis, when symptoms, standard echocardiographic parameters and ECG findings were included in the analysis, only relative apical longitudinal strain was significantly predictive of cardiac amyloidosis. The mechanism of apical sparing is not well understood. The authors speculated that amyloid deposition was less in the apex than the base and mid-ventricle as evidenced by less hypertrophy in the apex. Previous investigators using tissue Doppler-derived strain also suggested an apex-to-base gradient of longitudinal strain (15,16). Regional differences in circumferential strain also have been reported in cardiac amyloidosis (12). However, it remains unclear whether ‘apical sparing’ is diagnostic for cardiac amyloidosis, and further studies looking at the sensitivity and specific of this finding are needed. Moreover, the effect of disease stage or the type (amyloid light chain (AL) primary amyloidosis or transthyretin (TTR)) on this phenomenon has not been evaluated. However, the presence of ‘apical sparing’ should prompt consideration of the diagnosis of cardiac amyloidosis in patients with unknown origin of left ventricular hypertrophy, and may prove useful in avoiding underdiagnosis of cardiac amyloidosis (13).
CORONARY CT
Contrast-enhanced coronary CT angiography (CCTA) provides high-resolution images of the coronary arteries showing the severity and the location of significant stenosis and characteristics of atherosclerotic plaque. Because of its high diagnostic performance, CCTA has been increasingly used to exclude the presence of coronary stenosis (Table 1) (17-20). However, there is a paucity of data regarding the prevalence and characteristics of coronary atherosclerosis in asymptomatic patients with few risk factors for coronary disease. Kim et al (21) performed CCTA imaging in 2133 middle-aged asymptomatic patients who were classified as low risk by National Cholesterol Education Pro gram (NCEP) guidelines (22). Th ere were 243 persons (11.4%) with atherosclerosis plaques. Twenty-eight of them (1.3%) had a significant coronary stenosis, and 18 (0.8%) of them had significant coronary stenosis caused by non-calcified plaque (NCP). Most patients with significant stenosis had single-vessel disease and most of the significant lesions were located in the left anterior descending coronary artery. Notably, the majority of subjects with significant stenosis caused by NCP were young adults. Multivariate analysis clarified that male gender and LDL-cholesterol level were independent predictors of significant stenosis caused by NCP. Cardiac events occurred in four individuals during midterm follow-up (29.3±14.9 months). All four of these patients had atherosclerotic plaques, and three had significant NCP stenosis. From this study, we recognise that the prevalence of subclinical atherosclerosis is not negligible even in asymptomatic patients with low risk, especially in young adults. Although CCTA is not justified as a screening tool for asymptomatic patients by current guidelines, further research is needed to clarify if CCTA has the potential to identify high-risk patients who would otherwise be classified as low risk by NECP guidelines.
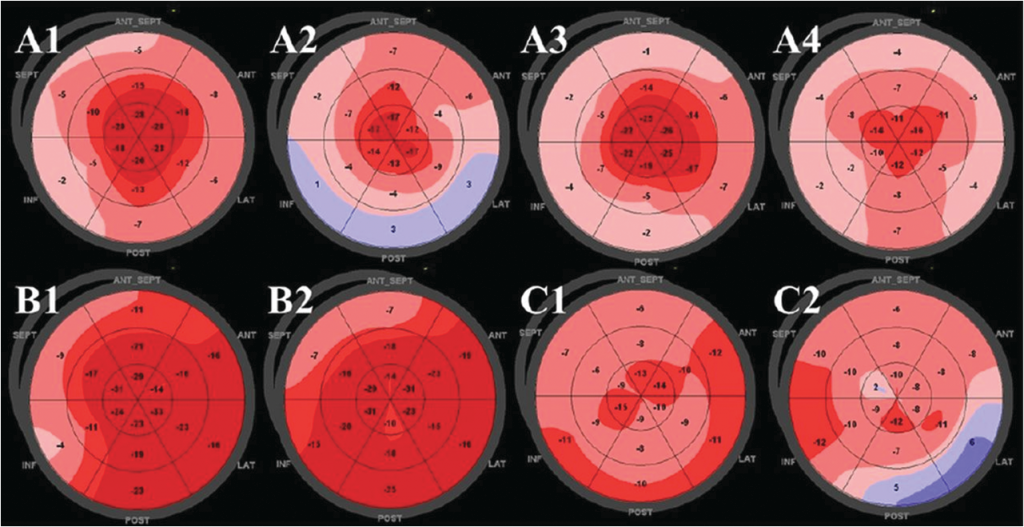
Figure 1.Representative two-dimensional speckle-tracking longitudinal strain patterns (‘bull’s eye plots’) for each subgroup. (A1–4) Apical sparing pattern in patients with cardiac amyloidosis. (B1,2) Isolated impairment of septal longitudinal strain in septal hypertrophic cardiomyopathy. (C1,2) Patchy reduction in longitudinal strain in left ventricular hypertrophy related to aortic stenosis. (Cited from ref. 14).
Table 1. Diagnostic performance of CT for anatomically obstructive stenosis in individuals without known CAD
| Sensitivity |
Specificity |
PPV |
NPV |
|
| ACCURACY | 94 | 83 | 48 | 99 |
| N=230, stable chest pain; no known CAD; no exclusion (CACS, HR, BMI); CAD prevalence 13% | ||||
| Meijboom et al | 99 | 64 | 85 | 97 |
| N=360, acute and stable chest pain; CAD prevalence 68% | ||||
| CorE64 | 85 | 90 | 91 | 83 |
| N=291, stable chest pain; no known and known CAD; exclusion CACS >600; CAD prevalence 56% | ||||
| Meijboom et al | 95 | 91 | 71 | 99 |
| N=415 (83), 20–80% pretest LK of CAD | ||||
BMI, Body Mass Index; CACS, Coronary Artery Calcification Score; CAD, coronary artery disease; HR, heart late; LK, likelihood; N, number; NPV, negative predictive value; PPV, positive predictive value (Cited from ref. 18).
There are many studies showing that CCTA can provide important prognostic information and risk stratification in patients with suspected coronary artery disease (23,24). However, most of the previous studies have focused on the general population, and limited data are available for age and gender-specific differences. To evaluate these differences in the incidence of coronary artery disease, CCTA was performed in 2432 patients with suspected coronary artery disease in the multicentre prospective registry study (25). Analysis was done in four subgroups stratified according to gender (male or female) and age (aged patients (41%) with normal CCTA results, 761 (31%) with non-significant coronary artery disease and 680 (28%) with significant coronary artery disease. During the follow-up (median 819 days), a cardiovascular event occurred in 59 (2.4%) with no gender-specific and age specific difference. CCTA results were predictive of the composite end point (non-fatal myocardial infarction and cardiac death) in male patients, both aged ≥60 years, and in female patients aged ≥60 years. However, in female patients aged were not predictive of adverse cardio- vascular events. Thus, while CCTA may be a valuable tool to rule out coronary artery disease, its prognostic value appears to be limited in women aged.
OPTICAL COHERENCE TOMOGRAPHY
Optical coherence tomography (OCT) has been extensively used recently as an intracoronary imaging method because of its high axial resolution ranging from 12 to 18 μm, compared with 150–200 μm for intravascular ultrasound (26). OCT is useful to visualise plaque microstructure, microvessels within coronary plaques, stents and neointimal changes inside stents (26,27). Intraplaque neovascularisation (NV), derived mainly from pre-existing vasa vasorum, has been recognised as an important process for the progression of atherosclerosis of larger vessels (28). However, investigation on coronary plaque NV has been limited. Tian et al (29) studied the significance of intraplaque NV in the coronary plaques using OCT. They analysed 92 culprit plaques and 203 non-culprit plaques
from 92 patients with unstable angina pectoris and 61 plaques from 25 patients with stable angina pectoris. A NV was defined as a small black hole within a plaque with a diameter of 50–300 μm that was present on at least three consecutive frames in pull-back images. The incidence of intraplaque NV was around 30% and not different among culprit and non-culprit lesions in patients with unstable and stable angina pectoris. However, among culprit lesions obtained from patients with unstable angina pectoris, plaques with NV had thinner fibrous cap, larger lipid core and higher incidence of thin cap fibroatheroma than those without NV. There was no significant difference in plaque characteristics between non-culprit lesions from unstable angina pectoris and lesions from stable angina pectoris. They found that the culprit plaques with NV had vulnerable features compared with those without NV in patients with unstable angina pectoris (Figure 2). Intraplaque
NV has dual effects on the plaque depending on the stage of the disease (30). At the early stage, it helps to supply nutrients and oxygen to the vessel wall and protects the plaque from ischaemic damage. However, at the late stage, with development of an imbalance between antiangiogenic and proangiogenic factors, the intraplaque NV becomes more immature and leaky, promoting the conversion of a stable plaque to an unstable plaque (30). Thus, intraplaque NV might aggravate destabilisation of plaques in patients with unstable angina pectoris.
MYOCARDIAL PERFUSION
Myocardial perfusion has been assessed by single photon emission CT (SPECT), positron emission tomography, perfusion MRI and contrast echocardiography. SPECT has been most popular, and an acceptable diagnostic capability to identify patients who have benefitted from revascularisation has been reported (31,32). However, the result of a diagnostic test may affect the subsequent referral for a more definitive test.
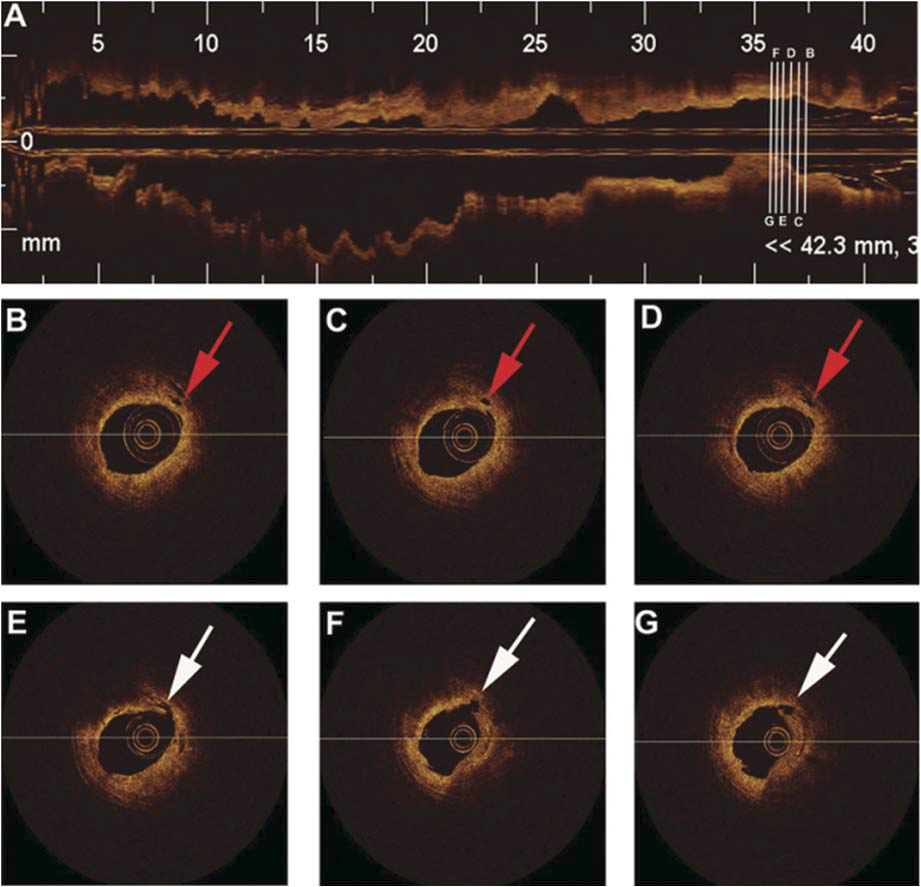
Figure 2.Representative optical coherence tomography images of ruptured plaque with neovascularisation (red arrows). The longitudinal pull-back image (A) of the vessel with the locations of five cross-sectional images (B–G). Neovascularisation (red arrows) were located at the shoulder region of plaque (B–D). Plaque rupture (white arrows) was visualised at the 7-o’clock position (E–G). Minimum lumen area site (G). (Cited from ref. 30).
When adjusted for referral bias, stress SPECT provided only 65% of sensitivity and 67% of specificity to identify patients with severe stenosis33. Moreover, there is a problem of limited spatial resolution and a lack of quantification. Multirow detector CT (MDCT) has better temporal and spatial resolution. It has been reported that MDCT can measure myocardial blood flow (MBF) using a model-based deconvolution method in a canine model of coronary stenosis34. Nakauchi et al (35) assessed the feasibility of this method to quantify myocardial perfusion in patients with acute myocardial infarction. They found tissue blood flow and tissue blood volume were significantly reduced in the infarcted myocardium compared with those in the non-infarcted myocardium. The defect area measured on the colour-coded tissue blood volume map correlated well with peak creatinine kinase level and SPECT defect score. In patients undergoing MDCT and MRI within a few days, the tissue blood flow measured with MDCT agreed well with that measured with MRI. Th is study demonstrated the feasibility of evaluating myocardial perfusion in a single CT as performed in clinical practice. Because the scan protocol is congruent with the baseline images for CCTA, it is possible to assess myocardial perfusion and coronary artery stenosis simultaneously in a single examination. CCTA is used to visualise coronary morphology. However, the haemodynamic significance of detected coronary stenosis cannot be evaluated by CT so that additional SPECT or myocardial perfusion MRI is needed. SPECT and myocardial perfusion MRI, however, are not helpful to assess coronary morphology. Clinically, these are relevant issues because it is important to identify the location of coronary stenoses that supply myocardium with demonstrated ischaemia as revascularisation leads to reduction of mortality and improved prognosis (36). Greif et al (37) report a study on myocardial perfusion imaging using CT imaging. The imaging protocol used a fast dual-source CT system, and evaluated coronary artery and myocardial perfusion with an adenosine stress test. Briefly, a dedicated parametric deconvolution technique was used, and from the maximum slope of the tissue time-attenuation curve, MBF was calculated (38). Fractional flow reserve (FFR) ≤0.80 measured by a pressure wire or lumen narrowing >90% was considered as haemodynamically significant coronary stenosis. CCTA detected all haemodynamically relevant stenosis (sensitivity 100%) while the specificity was 43.8% and diagnostic accuracy was 72%. Mean MBF was reduced in the myocardial segments pertaining to haemodynamically significant coronary stenosis. Sensitivity, specificity and diagnostic accuracy of CT myocardial perfusion imaging were 97%, 65.6% and 81.5%, respectively. The combination of CCTA and CT myocardial perfusion imaging demonstrated no significant further improvement in detection of haemodynamically significant stenosis compared with CTmyocardial perfusion imaging alone. Thus, CT myocardial perfusion imaging using a dual-source CT permits the detection of haemodynamically coronary artery stenosis with a moderate diagnostic accuracy. This method allows the simultaneous assessment of both coronary morphology and function non-invasively.
There are several other novel methods for physiologic assessment of coronary artery disease using CT (Table 2) (17). Th ere is a gradual diminution of contrast opacification from the proximal portion to the distal portion in the coronary artery. Transluminal contrast attenuation gradients (TAG) have been reported to decrease in accordance with Th rombolysis in Myocardial Infarction (TIMI) flow grade (39). However, this technique is dependent on a multiple factors such as left ventricular EF, contrast bolus rates, coronary flow velocity and contrast concentration and volumes. Owing to recent advances in computational flow dynamics, prediction of coronary flow and pressure, and thereby calculation of lesion-specific FFR (FFRCT, FFR CT technique) have been possible from typically acquired static CT images40. In a multicentre study with 150 vessels of intermediate stenosis from 252 patients, FFRCT was better than CCTA for the diagnosis of lesion-specific ischaemia (41). Thus, this method is promising to exclude patients with myocardial ischaemia non-invasively.
Table 2. Methods of physiologic assessment by CT
| Advantages |
Disadvantages |
|
| Transluminal contrast opacification gradients |
|
|
| Adverse plaque characteristics |
|
|
| CT perfusion |
|
|
| FFRCT |
|
|
CCTA, coronary CT angiography; FFRCT, fractional flow reserve CT technique. (Cited from ref. 18).
Hybrid SPECT/CCTA has been shown to have good performance in the diagnosis of significant coronary artery disease (Figure 3) (42). However, it has not been known whether there is a difference in the effect on the choice of treatment strategy between hybrid SPECT/ CCTA and SPECT plus coronary angi- ography. In a prospective study of 107 patients with stable angina pectoris, and an intermediate to high pretest likelihood of coronary artery disease (42), patients underwent an exercise or pharmacological stress SPECT study followed by CCTA. Coronary angiography was performed within 14 days of the SPECT/CCTA. The hybrid findings were categorised as matched, unmatched or normal. A matched finding was defined as an ischaemic SPECT defect in a myocardial territory sub-tended by a stenotic coronary artery. An unmatched finding was defined as an ischaemic SPECT defect without significant coronary lesion or a non-ischaemic SPECT finding with significant coronary lesion. A normal finding was defined as normal SPECT perfusion, with no significant coronary stenosis.
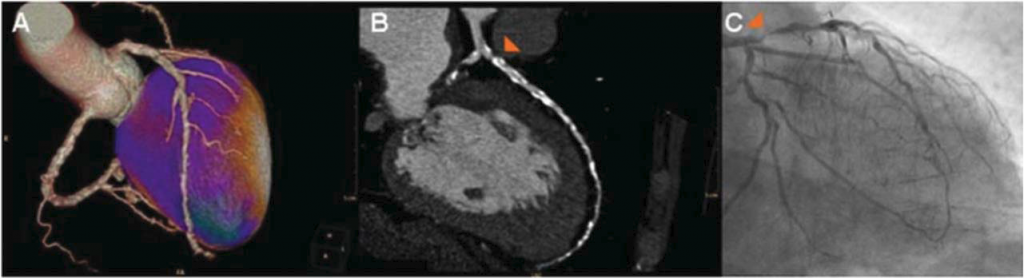
Figure 3. A 71-year-old male patient with typical anginal complaints, and a severe reversible perfusion defect anterior and apical on single photon emission CT (SPECT) (A, stress SPECT). Coronary CT angiography (CCTA) with evaluation was largely not possible due to severe coronary calcifications (coronary calcium score of 2279), although a severe stenosis in the proximal left anterior descending (LAD) artery was suspected (B). The presence of a significant stenosis in the proximal LAD was confirmed by invasive coronary angiogram (CA) (C). Based on evaluation of both hybrid SPECT/CCTA and SPECT and CA, the panel decided on percutaneous coronary intervention. (Cited from ref. 43).
The panel, consisting of two interventional cardiologists and one cardiothoracic surgeon, decided the percentage agreement of treatment decisions (no revascularisation, percutaneous coronary intervention or coronary artery bypass grafting) between hybrid SPECT/CCTA and SPECT and coronary angiography. The percentage agreement of treatment decisions in all patients on the necessity of revascularisation was 92%, and that in patients with matched, unmatched and normal hybrid SPECT/CCTA findings was 95%, 84% and 100%, respectively. The percent agreement stratified by method of revascularisation (percutaneous intervention or bypass surgery) was 72% for matched patients and 79% for unmatched patients. This study showed that a new work-up including hybrid SPECT/CCTA imaging, and a traditional workup including invasive coronary angiography, could reach similar treatment decisions.
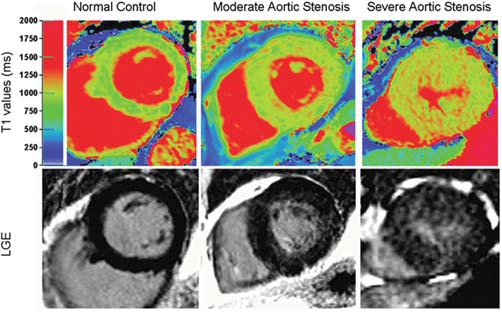
Figure 4. Top panel: colour maps of T1 values using shortened modified Look-Locker inversion in a mid-ventricular short-axis slice; bottom panel: the corresponding slice with late gadolinium enhancement imaging. The left -hand panel shows a normal volunteer (T1=944 ms). The middle panels show moderate aortic stenosis (AS) with moderate left ventricular hypertrophy (T1=951 ms). The right-hand panel shows severe AS with severe left ventricular hypertrophy (T1=1020 ms). (Cited from ref. 49).
Thus, patients with an intermediate to high pretest likelihood could be accurately deferred from, or indicated for revascularisation based on hybrid SPECT/CCTA, although we have to care about the radiation dose (43).
OTHERS
Myocardial fibrosis is found in various conditions, such as hypertension, ischaemic heart disease and cardiomyopathy. Because it directly relates to prognosis, non-invasive assessment of the degree of myocardial fibrosis is needed. Contrast-enhanced cardiovascular magnetic resonance (CMR) imaging has been extensively used to assess local fibrosis (44). Since the advent of transcatheter aortic valve implantation (TAVI), there is an increasing interest in AS. One of the determinants of prognosis of AS is expected to be myocardial fibrosis which occurs due to a long-standing pressure overload. Fairbairn et al (45) demonstrated that CMR measured myocardial fibrosis, decreased after 6 months in patients undergoing TAVI, although no effect was seen in patients undergoing surgical aortic valve replacement. Thus, CMR with postcontrast late gadolinium enhancement is useful, but it is time consuming, demands complex processing, and cannot be used in patients with severe renal impairment (46). Myocardial T1 values change with tissue composition (47). Bull et al (48) found that non-contrast CMR T1 mapping could identify myocardial fibrosis in patients with AS (Figure 4). They showed a significant correlation between T1 values and biopsy-quantified fibrosis. This method could be applied as a simple, non-invasive, non-contrast assessment of diffuse myocardial fibrosis in other cardiac diseases. Last, one article is taken up from nuclear cardiology (49). Cardiac sympathetic nerve function plays an important role in the pathophysiology, progression and the risk stratification and prediction of clinical out comes in chronic heart failure. Iodine-123 metaiodobenzylguanidine (123I-MIBG) is an analogue of norepinephrine, and cardiac 123I-MIBG scintigraphy has been used to assess myocardial sympathetic activity. Several publications have demonstrated clinical efficacy for cardiac 123I-MIBG imaging in heart failure patients.
Abnormal 123I-MIBG activity and augmentation of washout rate are closely related to deterioration of functional status, reduction of left ventricular EF and survival (50,51). To assess the prognostic value of cardiac 123I-MIBG scintigraphy to predict ventricular arrhythmias, Marshall et al conducted a prospective study in 27 patients with heart failure referred for implantable cardioverter defibrillator (ICD) implantation. There were 10 patients who experienced significant arrhythmic events at 16 months of median follow-up. These patients had lower early and late heart-to-mediastinum (H:M) ratio and higher 123I-MIBG SPECT defect scores than those without arrhythmic events. Early H;M ratio, late H:M ratio and defect score provided 60–78% of sensitivity, and 77–88% of specificity to predict arrhythmia. Thus, in patients with heart failure, cardiac 123I-MIBG imaging provides incremental prognostic information regarding the risk of future arrhythmia which may be helpful in informing the process of case selection for ICD therapy.
Competing interests: None.
Ethics approval. Provenance and peer review. Commissioned; externally peer reviewed.
References
1. Stanton T, Leano R, Marwick TH. Prediction of all-cause mortality from global longitudinal speckle strain: comparison with ejection fraction and wall motion scoring. Circ Cardiovasc Imaging 2009;2:356–64.
2. Cho GY, Marwick TH, Kim HS, et al. Global 2-dimensional strain as a new prognosticator in patients with heart failure. J Am Coll Cardiol 2009;54:618–24.
3. Ersbøll M, Valeur N, Mogensen UM, et al. Prediction of all-cause mortality and heart failure admissions from global left ventricular longitudinal strain in patients with acute myocardial infarction and preserved left ventricular ejection fraction. J Am Coll Cardiol 2013;61:2365–73.
4. Wang TJ, Larson MG, Levy D, et al. Temporal relations of atrial fibrillation and congestive heart failure and their joint influence on mortality: the Framingham Heart Study. Circulation 2003;107:2920–5.
5. Jang SJ, Kim MS, Park HJ, et al. Impact of heart failure with normal ejection fraction on the occurrence of ischaemic stroke in patients with atrial fibrillation. Heart 2013;99:17–21.
6. Zakeri R, Borlaug BA, McNuty SE, et al. Impact of atrial fibrillation on exercise capacity in heart failure with preserved ejection fraction. A RELAX trial ancillary study. Circ Heart Fail 2014;7:123–30.
7. Su HM, Lin TH, Hsu PC, et al. Global left ventricular longitudinal systolic strain as a major predictor of cardiovascular events in patients with atrial fibrillation. Heart 2013;99:1588–96.
8. Kusunose K, Yamada H, Nishio S, et al. Index-beat assessment of left ventricular systolic and diastolic function during atrial fibrillation using myocardial strain and strain rate. J Am Soc Echocardiogr 2012;25:953–9.
9. Lee CS, Lin TH, Hsu PC, et al. Measuring left ventricular peak longitudinal systolic strain from a single beat in atrial fibrillation: validation of the index beat method. J Am Soc Echocardiogr 2012;25:945–52.
10. Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography’s Guidelines and Standards Committee and the
Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr 2005; 18:1440–63.
11. Mor-Avi V, Lang RM, Badano LP, et al. Current and evolving echocardiographic techniques for the quantitative evaluation of cardiac mechanics: ASE/EAE consensus statement on methodology and indications. Endorsed by the Japanese Society of Echocardiography. J Am Soc Echocardiogr 2011;24:277–313.
12. Sun JP, Stewart WJ, Yang XS, et al. Diff erentiation of hypertrophic cardiomyopathy and cardiac amyloidosis from other causes of ventricular wall thickening by two-dimensional strain imaging echocardiography. Am J Cardiol 2009;103:411–15.
13. Guan J, Mishra S, Falk RH, et al. Current perspectives on cardiac amyloidosis. Am J Physiol Heart Circ Physiol 2012;302:H544–52.
14. Phelan D, Collier P, Th avendiranathan P, et al. Relative apical sparing of longitudinal strain using two-dimensional speckle-tracking echocardiography in both sensitive and specific for the diagnosis of cardiac amyloidosis. Heart 2012;98:1442–8.
15. Bellavia D, Pellikka PA, Abraham TP, et al. Evidence of impaired left ventricular systolic function by Doppler myocardial imaging in patients with systemic amyloidosis and no evidence of cardiac involvement by standard two-dimensional and Doppler echocardiography. Am J Cardiol 2008;101:1039–45.
16. Koyama J, Falk RH. Prognostic significance of strain Doppler imaging in light-chain amyloidosis. JACC Cardiovasc Imaging 2010;3:333–42.
17. Min JK, Castellanos J, Siegel R. New frontiers in CT angiography: physiologic assessment of coronary artery disease by multidetector CT. Heart 2013;99:661–8.
18. Budoff MJ, Dowe D, Jollis JG, et al. Diagnostic performance of 64-multidetector row coronary computed tomographic angiography for evaluation of coronary artery stenosis in individuals without known coronary artery disease: results from the prospective multicenter ACCURACY (Assessment by Coronary Computed Tomographic Angiogaphy of Individuals Undergoing Invasive Coronary Angiography) atrial. J Am Coll Cardiol 2008;52:1724–32.
19. Meijboom WB, Meijs MF, Schuijf JD, et al. Diagnostic accuracy of 64-slice computed tomography coronary angiography: a prospective, multicenter, multivendor study. J Am Coll Cardiol 2008;52:2135–44.
20. Miller JM, Rochitte CE, Dewey M, et al. Diagnostic performance of coronary angiography by 64-row CT. N Engl J Med 2008;359:2324–36.
21. Kim KJ, Choi SI, Lee MS, et al. Th e prevalence and characteristics of coronary atherosclerosis in asymptomatic subjects classified as low risk based on traditional risk stratification algorithm: assessment with coronary CT angiography. Heart 2013;99:1113–17.
22. Panel NCEPE. Third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) final report. Circulation 2002;106:3143–421.
23. Aldrovandi A, Maff ei E, Palumbo A, et al. Prognostic value of computed tomography coronary angiography in patients with suspected coronary artery disease: a 24-month follow-up study. Eur Radiol 2009;19:1653–60.
24. Hadamitzky M, Freissmuth B, Meyer T, et al. Prognostic value of coronary computed tomographic angiography for prediction of cardiac events in patients with suspected coronary artery disease. JACC Cardiovasc Imaging 2009;2:404–11.
25. Yiu KH, de Gaaf FR, Schuijf JD, etal. Age- and gender-specific differences in the prognostic value of CT coronary angiography. Heart 2012;98:232–7.
26. Bezerra HG, Costa MA, Guagliumi G, et al. Intracoronary optical coherence tomography: a comprehensive review. Clinical and research applications. J Am Coll Cardiol Intv 2009;2:1035–46.
27. Uemura S, Ishigami KI, Soeda T, et al. Th in-cap fibroatheroma and microchannel findings in optical coherence tomography correlate with subsequent progression of coronary atheromatous plaques. Eur Heart J 2012;33:78–85.
28. Moreno PR, Purushothaman KR, Fuster V, et al. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: implications for plaque vulnerability. Circulation 2004;110:2032–8.
29. Tian J, Hou J, Xing L, et al. Significance of intraplaque neovascularization for vulnerability: optical coherence tomography study. Heart 2012;98:1504–9.
30. Virmani R, Kolodgie FD, Burke AP, et al. Atherosclerotic plaque progression and vulnerability to rupture: angiogenesis as a source of intraplaque hemorrhage. Arterioscler Thormb Vasc Biol 2005;25:2054–61.
31. Shaw LJ, Berman DS, Maron DJ, et al. Optimal medical therapy with or without percutaneous coronary intervention to reduce ischemic burden: results from the Clinical Outcomes Utilizing Revascularization and Aggressive Drug Evaluation (COURAGE) trial nuclear substudy. Circulation 2008;117:1283–91.
32. Hachamovitch R, Rozanski A, Shaw LJ, et al. Impact of ischaemia and scar on the therapeutic benefit from myocardial revascularization vs. medical therapy among patients understanding stress-rest myocardial perfusion scintigraphy. Eur Heart J 2011;32:1012–24.
33. Miller TD, Hodge DO, Christian TF, et al. Eff ects of adjustment for referral bias on the sensitivity and specificity of single photon emission computed tomography for the diagnosis of coronary artery disease. Am J Med 2002;112:290–7.
34. George RT, Jerosch-Herold M, Silva C, et al. Quantification of myocardial perfusion using dynamic 64-detector computed tomography. Invest Radiol 2007;42:815–22.
35. Nakauchi Y, Iwanaga Y, Ikuta S, et al. Quantitative myocardial perfusion analysis using multi-row detector CT in acute myocardial infarction.Heart 2012;98:566–72.
36. Pijls NH, Fearon WF, Tonio PA, et al. Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2-year follow-up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol 2010;56:177–84.
37. Greif M, von Ziegler F, Bamberg F, et al. CT stress perfusion imaging for detection of haemodynamically relevant coronary stenosis as defined by FFR. Heart 2013;99:1004–11.
38. Bamberg F, Klotz E, Flohr T, et al. Dynamic myocardial stress perfusion imaging using fast dual source CT with alternating table positions: initial experience. Eur Radiol 2010;20:1168–73.
39. Choi JH, Min JK, Labounty TM, et al. Intracoronary transluminal attenuation gradient in coronary CT angiography for determining coronary artery stenosis. JACC Cardiovasc Imaging 2011;4:1149–57.
40. Kim HJ, Vignon-Clementel IE, Coogan JS, et al. Patient-specific modeling of blood flow and pressure in human coronary arteries. Ann Biomed Eng 2010;38:3195–209.
41. Nakazato R, Park HB, Berman DS, et al. Noninvasive fractional flow reserve derived from computed tomography angiography for coronary lesions of intermediate stenosis severity. Results from the De- FACTO study. Circ Cardiovasc Imaging 2013;6:881–9.
42. Schaap J, de Groot JAH, Nieman K, et al. Hybrid myocardial perfusion SPECT/CT coronary angiography and invasive coronary angiography in patients with stable angina pectoris lead to similar treatment decisions. Heart 2013;99:188–94.
43. Brix G, Nekolla EA, Borowski M, et al. Radiation risk and protection of patients in clinical SPECT/CT. Eur J Nucl Med Mol Imaging 2014;41(suppl 1):S125–36.
44. Kwong RY, Farzaneh-Far A. Measuring myocardial scar by CMR. JACC Cardiovasc Imaging 2011;4:157–60.
45. Fairbairn TA, Steadman CD, Mather AN, et al. Assessment of valve haemodynamics, reverse ventricular remodeling and myocardial fibrosis following transcatheter aortic valve implantation compared to surgical aortic valve replacement: a cardiovascular magnetic resonance study. Heart 2013;99:1185–91.
46. Mewton N, Liu CY, Croisille P, et al. Assessment of myocardial fibrosis with cardiovascular magnetic resonance. J Am Coll Cardiol 2011; 57:891–903.
47. Messroghli DR, Niendorf T, Schultz-Menger J, et al. T1mapping in patients with acute myocardial infarction. J Cardiovasc Magn Reson 2003;5:353–9.
48. Bull S, White SK, Piechnik SK, et al. Human non-contrast T1values and correlation with histology in diff use fibrosis. Heart 2013;99:932– 7.
49. Marshall A, Cheetham A, George RS, et al. Cardiac iodine-123 metaiodo benzylguanidine imaging predicts ventricular arrhythmia in heart failure patients receiving an implantable cardioverter-defibrillator for primary prevention. Heart 2012;98:1359–65.
50. Jacobson AE, Senior R, Cerqueira MD, et al. Myocardial iodin-123 meta-iodobenzylguanidine imaging and cardiac events in chronic heart failure. Results of the progressive ADMIRE-HF (AdreView myocardial imaging for risk evaluation in heart failure) study. J Am Coll Cardiol 2010;55:2212–21.
51. Nakata T, Nakajima K, Yamashina S, et al. A pooled analysis of multicenter cohort studies of I-123-mIBG cardiac sympathetic innervation imaging for assessment of long-term prognosis in chronic heart failure. JACC Cardiovasc Imaging 2013;6:772–84.
 This work is licensed under a
This work is licensed under a