Download PDF
https://doi.org/10.47803/rjc.2020.30.4.629
Dan Musat1, Mark Preminger1, Luisito Banzon1, Catherine Hanson1, Yanire Gonzales1, Susan Oliveros1, Suneet Mittal1
1 Department of Cardiovascular Services, Snyder Center for Comprehensive Atrial Fibrillation, Valley Health System, Paramus, NJ
Pacemaker therapy has become standard of care for symptomatic brady-arrhythmias due to irreversible heart block and sinus node dysfunction. The pacemaker technologies have evolved significantly, with many features that improve battery life, and remote monitoring with automatic checks of leads integrity and assuring good function, like automatic sensing and pacing threshold evaluations and self-adjustments based on certain algorithms. The case presentation involves appropriate function of one of these algorithms however with unintended consequences. Patient is a pleasant 84-year-old man with hypertension, controlled with medications (bisoprolol, amlodipine, losartan) which led to chronic kidney disease stage III, paroxysmal atrial fibrillation and a prior stroke for which patient is currently on anticoagulation with apixaban. He also has a history of trifascicular block (1st degree AV block, right bundle branch block and left anterior fascicular block), who in 2008 presented at the hospital with recurrent episodes of syncope. At that time a diagnostic electrophysiology study demonstrated severe infrahisian conduction disease and he underwent insertion of a Medtronic dual chamber pacemaker in April 2008. He underwent generator change in June 2019, with a Medtronic Advisa pacemaker, when the leads were found to function normally, with the knowledge that his right ventricular (RV) pacing threshold was chronically mildly elevated at 1.75-2.0 V at 0.4 msec (Figure 1). The atrial and ventricular adaptive capture management was programmed on in the previous Adapta pacemaker, with 2 X amplitude margin and minimum adapted amplitude of 1.5 V for atrial lead and 2.0 V for ventricular lead. The patient presented to hospital in April 2020, 10 months after generator change, with complaints of episodes of dizziness, and near syncopal, and syncopal episodes lasting for seconds, not associated with exercise, occurring even when lying in bed. On presentation to the emergency room the ECG showed sequential atrial and ventricular pacing (Figure 2), however shortly thereafter, on telemetry, there were episodes with loss of ventricular capture and pauses up to 10 to 12 seconds (Figure 3 A). Patient was severely symptomatic during these episodes with near syncope. A Carelink Express interrogation of the pacemaker was performed by the emergency room staff, with automatic interrogation and transmission of data to Medtronic company, that was remotely evaluated by one of the company device technicians, with results being called back and faxed to the emergency room physician. It was noticed that there was failure to capture on the transmission EGM (Figure 3 B), however, the interrogation showed normal leads parameters, including stable impedances and auto pacing thresholds (Figure 3 C) Chest X-Ray showed normal leads position (Figure 4). It was clear that this was a pacemaker pacing output problem, as revealed by the Carelink Express interrogation. The automatic interrogation showed an automatic threshold of 0.75 V @ 0.4 msec and RV pacing output of 2V @ 0.4 msec. There was no inhibition of the impulse delivery and the right ventricular impulse was delivered at the appropriate timing, sequentially after the atrial sensed event. Lead impedances were stable, both bipolar and unipolar, no noise was seen, short intervals count (suggestive of lead fracture) was 0. Laboratories showed normal potassium and magnesium. After several minutes, consistent ventricular capture was seen and patient became asymptomatic. Interrogation performed by electrophysiologist demonstrated wide variations of the automatic pacing threshold determinations ranging from 0.625V up to 2.25V. At the pacemaker generator change in June 2019, the initial right ventricular pacing output was programmed at 3.5V @ 0.4msec for RV pacing threshold of 1.75V @ 0.4msec demonstrated during the procedure and adaptive capture management algorithm was turned on. However, the default lower limit for adaptive output of 2.0V @ 0.4msec was not increased, mimicking previous pacemaker programming. Over the past several months, the remote transmissions showed that the patient has become pacemaker dependent. For Medtronic pacemakers the pacing threshold is checked automatically daily in early morning hours and not on a beat-to-beat basis. Therefore, in the morning of the presentation, the automatic pacing threshold was 0.75V @ 0.4 msec and pacemaker adapted it automatically to the lowest default output of 2.0V @ 0.4 msec. Most likely in the afternoon the pacing threshold was intermittently higher than 2.0V @ 0.4 msec, leading to non-captured beats associated with the symptoms he was experiencing. Upon manual interrogation, the pacing threshold was back to 1V @ 0.4 msec. The pacemaker was reprogrammed to auto-capture but with the lowest adaptive output to 3.5V @ 0.4 msec, with good safety margin. In follow-up patient had not any other symptoms.
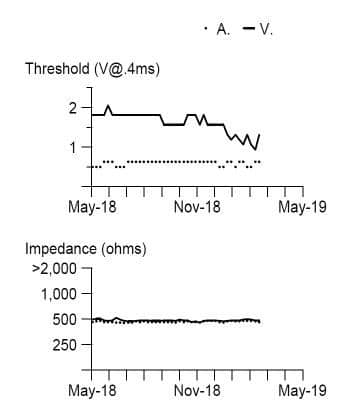
Figure 1. Prior to generator change leads pacing threshold and impedance long term trends.
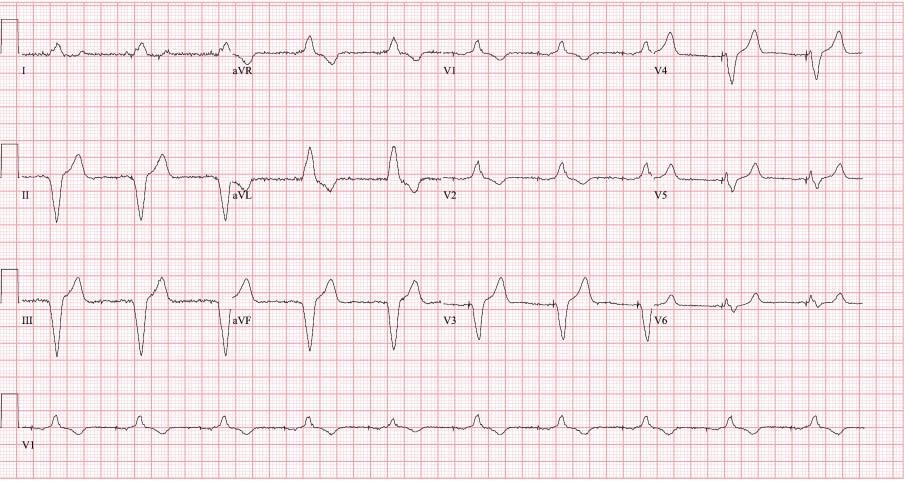
Figure 2. Presenting ECG.
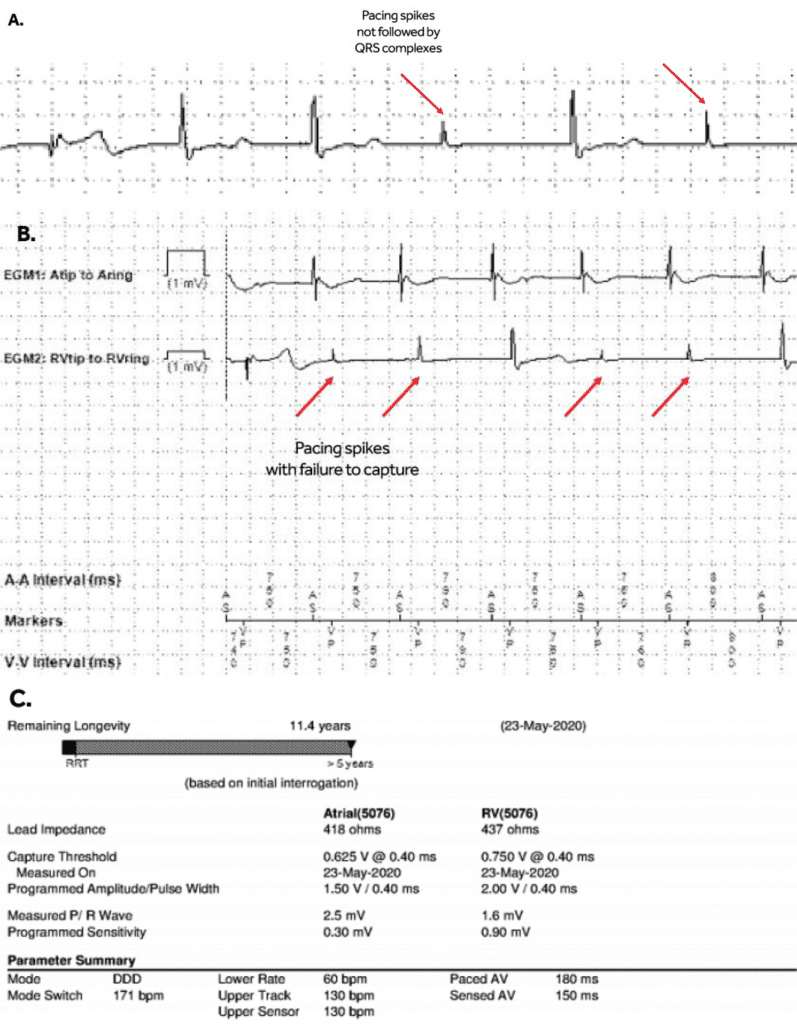
Figure 3. A. Telemetry snapshot; B. Medtronic Carelink Express initial interrogation – presenting rhythm; C. Medtronic Carelink Express initial interrogation – pacemaker parameters (low R wave sensing amplitude, 1.6 mV, likely sensing intrinsic ventricular escape beats).
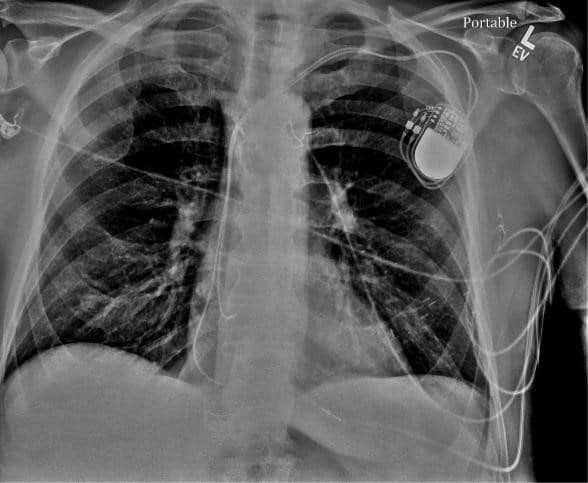
Figure 4. Chest XRay – normal leads position.
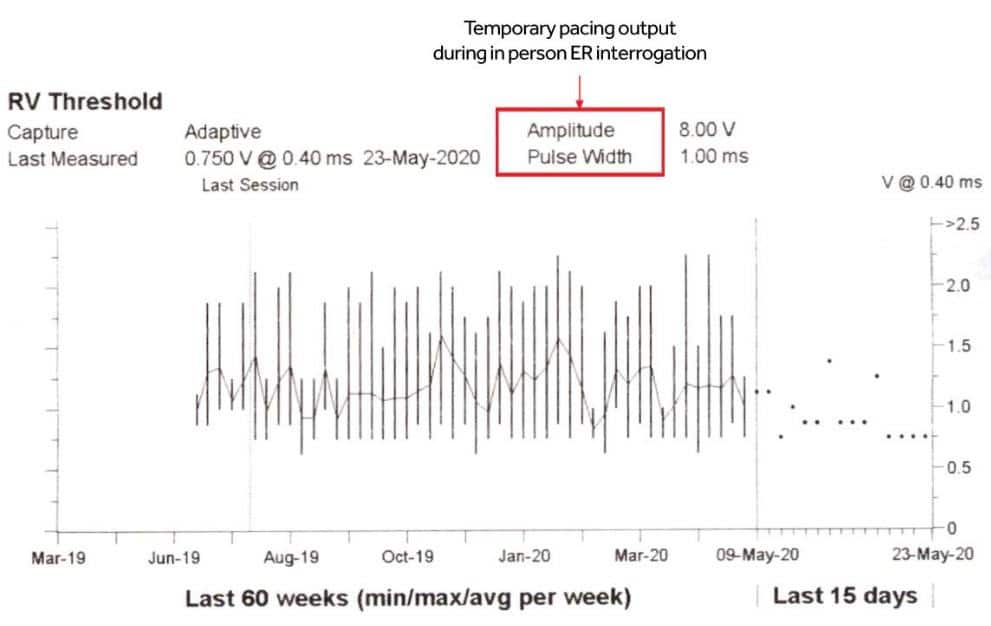
Figure 5. Right ventricular pacing auto-thresholds trend – ranging from 0.625V and 2.25V.
DISCUSSION
The algorithms for auto evaluation of pacemakers have become widely used, especially in the current era of remote monitoring, allowing monitoring from home with fewer in-office visits1. Automatic threshold algorithm checks the pacing threshold and sets the pacing output 0.5V or 1V above the threshold. The automatic threshold evaluation and pacing output adjustment has led to significantly increase in battery life2. The automatic pacing threshold algorithms can be widely implemented, in more than 90% of the patients, failure being due to high pacing thresholds, perceived problems with automatic measurements including frequent PVCs, inadequate sensing/sensitivity, etc2. They proved to have high accuracy, with very low difference of 0.07V between automatic threshold and manual threshold2. Two of the device companies, Abbott (former St. Jude Medical) and Boston Scientific provide beat-to-beat threshold pacing and backup pacing, with Medtronic and Biotronik having the pacing threshold checked on a daily basis. Variations in day-to-day pacing threshold usually are low, of 0.1V, however a threshold of > 0.4V could be seen in up to 10% of patients3. For patients with large variations, a beat-to-beat auto pacing threshold capable device would be ideal. However, for the other devices either a fixed pacing output with an adequate safety margin taking in consideration the highest threshold should be considered, or auto-capture threshold with the lowest adapted pacing output higher than the highest registered automatic threshold. In our patient, the problem was that minimal adapted pacing amplitude was not programmed accordingly to assure a safety margin, and we have opted for the second option with appropriate pacing safety margin. The patient has not had any other symptoms since the reprogramming. The loss of pacing capture is rare with contemporary pacemakers. Causes of acute increase in pacing threshold include metabolic and electrolytes derangements or ischemia4. There is one other case report involving failure of the auto-threshold pacing algorithm reported by Kishihara et al, involving a Medtronic Advisa pacemaker5. The episodes of syncope and non-capture pacing occurred 4 years from the implant. The authors report that although a wide range of autocapture thresholds is seen in their case as well (0.625V– 1.625V), for them it still remains unclear why the failure to capture occurred as pacing output was set for 3V @ 0.4 msec. They report an interesting finding of increasing ventricular pacing threshold with increase in AV delay, from 1.5V @ 0.4 msec at an AV delay of 80 msec up to 2.75V @ 0.4 msec at an AV delay of 220 msec during pacing at a rate of 80 ppm. They speculate that the differences could be due to either dynamic change in lead-tissue contact, or the chronic use of steroids in this patient with systemic lupus erythematosus, leading to cardiac fibrosis. Our patient had demonstrated, for almost a year prior, a large variation of automatic pacing threshold. The question is why this was not a problem for our patient since pacemaker generator change? There are 2 possible explanations: 1. Patient only recently became completely pacemaker dependent, so even if pacing failure was occurring previously, he was asymptomatic with intrinsic rhythm. 2. Patient would not have such high pacing thresholds during the periods when the output was set at 2.0V @ 0.4 msec.
CONCLUSION
Capture management with automatic pacing threshold and adjustment is a very useful feature in the pace-makers and significantly improves battery life. This feature should be implemented carefully, with safety margins in mind, in devices not able to provide beat-to beat feedback and backup pacing.
Conflict of interest: none declared.
References:
1. Slotwiner D, Varma N, Akar JG, et al.: HRS Expert Consensus State-ment on remote interrogation and monitoring for cardiovascular im-plantable electronic devices. Heart Rhythm 2015; 12:e69–e100.
2. KOPLAN BA, GILLIGAN DM, NGUYEN LS, et al.: A Randomized Trial of the Effect of Automated Ventricular Capture on Device Lon-gevity and Threshold Measurement in Pacemaker Patients. Pacing Clin Electrophysiol John Wiley & Sons, Ltd, 2008; 31:1467–1474.
3. PECORA D, MORANDI F, LICCARDO M, et al.: Performance of a Ventricular Automatic-Capture Algorithm in a Wide Clinical Setting. Pacing Clin Electrophysiol John Wiley & Sons, Ltd, 2008; 31:1546– 1553.
4. Sonou A, Adjagba PM, Hounkponou M, et al.: Perte de capture par ischémie myocardique: à propos d’un cas. Ann Cardiol Angéiologie 2017; 66:55–57.
5. Kishihara J, Niwano S, Fukaya H, et al.: Pacing failure caused by auto-matic pacing threshold adjustment system. J Arrhythmia John Wiley & Sons, Ltd, 2017; 33:637–639.
 This work is licensed under a
This work is licensed under a