Cristina Bulei1, Aura Popa1, Claudia Folescu1, Marinela Serban1, Anca Mateescu1, Serban State1, Carmen Ginghina1
1 „C.C Iliescu” Emergency Institute for Cardiovascular Diseases, Bucharest, Romania
Abstract: We are presenting the case of a woman, 37 years old, former smoker, who was admitted for palpitations lasting a few seconds, accompanied by shortness of breath and dry cough. The patient complained about shortness of breath induced by ordinary physical activity, paroxysmal nocturnal dyspnoea and chest pain that occurred at rest and disappeared in approximately 5 minutes after its onset. She had been diagnosed a year previously with rheumatic mitral valve disease consisting of moderate stenosis and mild regurgitation. The young woman had a normal menstrual cycle and three children aged between 7 and 12 years old (all the pregnancies were full-term). When taking the social history, the patient revealed that she had grown up in an orphanage and at the age of 18, soon after leaving it, she was diagnosed with acute rheumatic fever. The patient’s treatment before admission consisted of an anti-vitamin K anticoagulant, and antiarrhythmic drugs – amiodarone and a beta blocker. On physical examination, the patient was afebrile and had a normal Body Mass Index. The auscultation revealed rhythmic heart sounds, an early diastolic opening snap and a mitral diastolic rumble near the apex. There were no signs of systemic or pulmonary congestion. The blood tests detected elevated erythrocyte sedimentation rate (ESR) and creatine kinase –MB (CK-MB); the rest of the laboratory values were normal, including that of the B-type natriuretic peptide (BNP). The ECG showed sinus rhythm and left atrial enlargement which was visible on the otherwise normal chest x-ray. Transthoracic echocardiography revealed mitral valve disease with moderate stenosis (mitral valve area, MVA, estimation by PHT: 1.4 cm2 – Figure 1, mean gradient: 6 mmHg – Figure 2) and mild regurgitation with an eccentric jet towards the posterior left atrial wall. The dimensions and the systolic function of both ventricles were normal (LVEF 65%), but the left atrium was severely enlarged (Figure 3). Transoesophageal echocardiography confi rmed the mitral valve disease with signifi cant mitral stenosis (MVA by planimetry: 1.1 cm2 , MVA by PHT: 1.3 cm2 ) and mild regurgitation. It also allowed a better appraisal of the mitral valve morphology: fusion of the commissures (Figure 4), small calcifi ed nodules on the anterior mitral leafl et, a dome-like opening of the valve and also an assessment of the Wilkins score of 7 (leafl et mobility: 2, subvalvular apparatus involvement: 2, leafl et thickening: 2, valve calcifi cation: 1). There was no evidence of thrombi either in the enlarged left atrium or in the left atrial appendage. Both echographic examinations revealed mild aortic regurgitation and the lack of diagnostic criteria for pulmonary hypertension (estimated PASP: 31 mmHg). After discussing the therapeutic options with the patient, we decided for a percutaneous balloon mitral valvuloplasty in the case of a young symptomatic patient with a signifi cant mitral stenosis, with favourable valve characteristics both hemodynamically and morphologically. During the two months preceding the intervention, the patient was prescribed a beta blocker, an anti-vitamin K anticoagulant, a loop diuretic. As this was the case of a young woman with long-term necessity of antiarrhythmic therapy, considering the possible side effects of amiodarone, it was replaced with propafenone. After the balloon valvuloplasty, the area of the mitral valve was 1.9 cm2 . A week after the intervention, the transthoracic echocardiography revealed an estimated MVA of 1.8 cm2 by planimetry (Figure 6) and 1.6 cm2 by PHT, a mean gradient of 4 mmHg (Figure 7) and a slight decrease in the volume of the left atrium, which remained nevertheless severely enlarged. The mitral regurgitation was mild and the estimated PASP receded to 17 mmHg. The patient did not tolerate the propafenone therapy, complaining about dyspnoea at rest after taking the drug. At discharge, the patient claimed amelioration of the symptoms and was prescribed a beta blocker, anti-vitamin K anticoagulant, low dose loop diuretic and secondary prophylaxis for rheumatic fever – penicillin V. At the following examinations, the necessity of an antiarrhythmic therapy remains to be reassessed. Keywords: rheumatic mitral stenosis, percutaneous balloon mitral valvuloplasty.
INTRODUCTION
Unlike other valvular lesions which have many etiologies, mitral stenosis, either isolated or associated with other valvulopathies, is almost exclusively secondary to rheumatic heart disease (RHD). Acute rheumatic fever (ARF) is a systemic infl ammatory disease of the connective tissue, characterised by fi brous degeneration of the connective tissue due to an abnormal immune response to a group A beta-haemolytic streptococcal pharyngotonsillits. It affects mainly the heart, joints, skin, subcutaneous tissue and the central nervous system1 . The destructive effect on the heart valves leads to RHD and severe haemodynamic perturbation, followed by heart failure and other complications such as stroke and infectious endocarditis2 . Rheumatic valve lesions are among the most frequent non-communicable diseases in low and middle income countries and RHD is annually responsible for 250000 premature deaths worldwide3 . ARF and RHD can be considered the consequences of poverty and social inequality. Although the incidence of ARF is declining in developed countries (due to better nutrition, hygiene and living conditions, improved accessibility to health care services and to antibiotic therapy1 , 75% of the children under 15 years old reside in countries where RHD is endemic4.
CASE PRESENTATION
We are presenting the case of a young woman, 37 years old, former smoker, who was admitted for palpitations lasting a few seconds, accompanied by shortness of breath and dry cough. The patient complained about shortness of breath induced by ordinary physical activity, paroxysmal nocturnal dyspnoea and chest pain that occurred at rest and disappeared in approximately 5 minutes after its onset. She had been diagnosed a year previously with rheumatic mitral valve disease consisting of moderate stenosis and mild regurgitation and with paroxysmal atrial fi brillation. The young woman had a normal menstrual cycle and three clinically healthy children (without ARF or valvulopathies) aged between 7 and 12 years old (all the pregnancies were full-term). When taking the social history, the patient revealed that she had grown up in an orphanage and at the age of 18, soon after leaving it, she was diagnosed with acute rheumatic fever. The patient’s treatment before admission consisted of an anti-vitamin K anticoagulant, and antiarrhythmic drugs – amiodarone and a beta blocker. On physical examination, the patient was afebrile and had a normal Body Mass Index. The auscultation revealed rhythmic heart sounds, an early diastolic opening snap and a mitral diastolic rumble near the apex. There were no signs of systemic or pulmonary congestion. The blood tests detected elevated erythrocyte sedimentation rate (ESR) and creatine kinase – MB (CKMB); the rest of the laboratory values were normal, including that of the B-type natriuretic peptide (BNP). The ECG showed sinus rhythm and left atrial enlargement which was visible on the otherwise normal chest x-ray. Transthoracic echocardiography revealed mitral valve disease with moderate stenosis (mitral valve area, MVA, estimation by PHT: 1.4 cm2 – Figure 1, mean gradient: 6 mmHg – Figure 2) and mild regurgitation with an eccentric jet towards the posterior left atrial wall. The dimensions and the systolic function of both ventricles were normal (LVEF 65%), but the left atrium was severely enlarged (Figure 3). Transoesophageal echo cardiography confi rmed the mitral valve disease with signifi cant mitral stenosis (MVA by planimetry: 1.1 cm2 , MVA by PHT: 1.3 cm2 ) and mild regurgitation. It also allowed a better appraisal of the mitral valve morphology: fusion of the commissures (Figure 4), small calcifi ed nodules on the anterior mitral leafl et, a dome-like opening of the valve and also an assessment of the Wilkins score of 7 (leafl et mobility: 2, subvalvular apparatus involvement: 2, leafl et thickening: 2, valve calcification: 1). There was no evidence of thrombi either in the enlarged left atrium or in the left atrial appendage. Both echographic examinations revealed mild aortic regurgitation and the lack of diagnostic criteria for pulmonary hypertension (estimated PASP: 31 mmHg). After discussing the therapeutic options with the patient, we decided for a percutaneous balloon mitral valvuloplasty in the case of a young symptomatic patient with a signifi cant mitral stenosis, with favorable valve characteristics both hemodynamically and morphologically. During the two months preceding the intervention, the patient was prescribed a beta blocker, an anti-vitamin K anticoagulant, a loop diuretic. As this was the case of a young woman with long-term necessity of antiarrhythmic therapy, considering the possible side effects of amiodarone, it was replaced with propafenone. After the balloon valvuloplasty, the area of the mitral valve was 1.9 cm2 . A week after the intervention, the transthoracic echocardiography revealed an estimated MVA of 1.8 cm2 by planimetry (Figure 6) and 1.6 cm2 by PHT, a mean gradient of 4 mmHg (Figure 7) and a slight decrease in the volume of the left atrium, which remained nevertheless severely enlarged. The mitral regurgitation was mild and the estimated PASP receded to 17 mmHg. The patient did not tolerate the propafenone therapy, complaining about dyspnoea at rest after taking the drug. At discharge, the patient claimed amelioration of the symptoms and was prescribed a beta blocker, antivitamin K anticoagulant, low dose loop diuretic and secondary prophylaxis for rheumatic fever – penicillin V. At the following examinations, the necessity of an antiarrhythmic therapy remains to be reassessed.
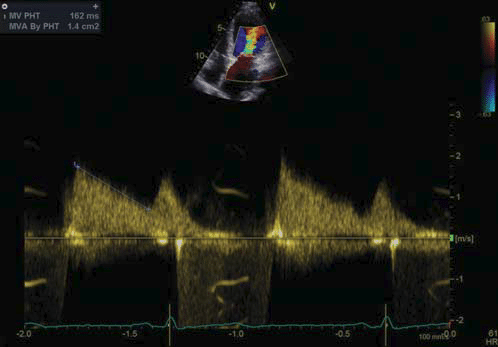
Figure 1. Transthoracic echocardiography, apical four chamber view continuous wave Doppler at the level of the transmitral fl ow – PHT: 162 ms, MVA by PHT: 1.4 cm2 .
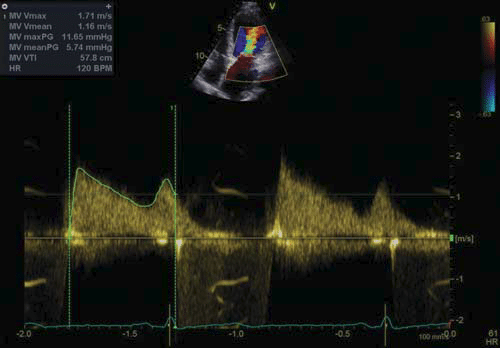
Figure 2. Transthoracic echocardiography, continuous wave Doppler apical four chamber view, transmitral fl ow – maximum gradient: 11.6 mmHg, mean gradient: 5.6 mmHg.
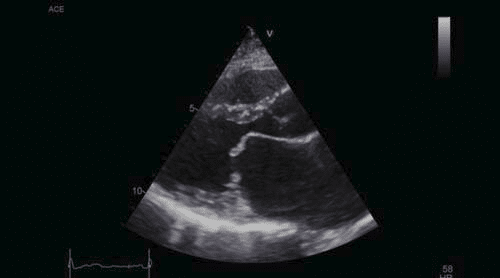
Figure 3. Transthoracic echocardiography, parasternal long axis view – severe enlargement of the left atrium, limited opening of the mitral valve, diastolic doming of the mitral anterior leaflet.
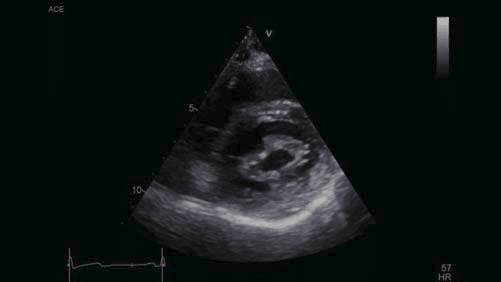
Figure 4. Transthoracic echocardiography, parasternal short axis view at the level of the mitral valve – limited opening of the mitral valve, thickened mitral leafl ets, MVA by planimetry 1.3 cm2 .
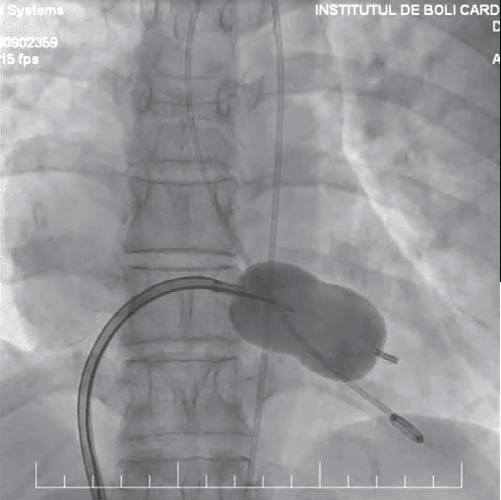
Figure 5. Cardiac fluoroscopy, posteroanterior view – the balloon is placed at the level of the mitral valve; by inflating the middle part of the balloon, the fused commissures are fractured.
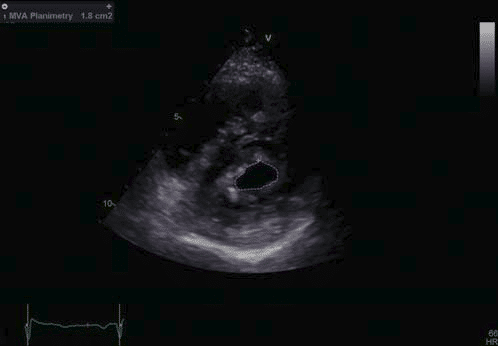
Figure 6. Transthoracic echocardiography, parasternal short axis view at the level of the mitral valve – limited opening of the mitral valve, thickened mitral leaflets, MVA by planimetry 1.8 cm2 .
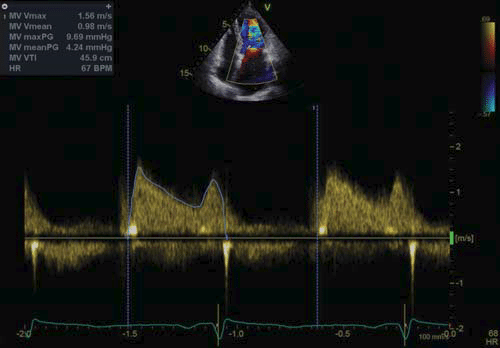
Figure 7. Transthoracic echocardiography, apical four chamber view continuous wave Doppler at the level of the transmitral flow – maximum gradient: 9 mmHg, mean gradient: 4 mmHg. The mitral stenosis is mild after the percutaneous balloon mitral valvuloplasty
DISCHARGE DIAGNOSIS
Percutaneous balloon mitral valvuloplasty for moderate rheumatic mitral stenosis
Heart failure NYHA class III
Paroxysmal atrial fibrillation
Severely enlarged left atrium
Mild mitral regurgitation
Mild aortic regurgitation
Mild tricuspid regurgitation
Mild hyperkalemia – corrected
Bilateral hypermetropia
Compound hyperopic astigmatism and mild amblyopia – left eye
DISCUSSIONS
The most frequent consequence of ARF is RHD which usually appears 10 to 20 years after the initial rheumatic fever episode. RHD is the most common cause for valvular lesions worldwide5 . Chronic valvular injury as a result of ARF was evaluated in the pre-antibiotic era in a prospective study involving 1000 children and adolescents. At the end of the twenty-year follow-up period, 70 percent of participants had at least one valvulopathy and in over 65 percent of cases the clinical evidence of the valvular heart disease was present shortly after recovering from the initial episode of ARF6 . Mitral valve involvement is the most common, while aortic valvular lesions are seen in 20-30 percent of patients with RHD. The tricuspid valve is also often affected, but the lesion is frequently subclinical until surgery becomes necessary. The incidence and the pattern of the mitral valve injuries in RHD vary in consonance with age – patients under 30 years old present predominantly with pure mitral regurgitation, mitral stenosis usually develops in adult middle aged subjects, whereas the mixed mitral valve disease becomes dominant in older patients7 . Isolated mitral stenosis is present in approximately 25 percent of subjects with RHD, while 40 percent of patients are diagnosed with mixed mitral valve disease2 . The patient was diagnosed with ARF when she was 18 years old and until that age she had been brought up in an orphanage in diffi cult living conditions. Overcrowding, collective living, poor hygiene, malnutrition and limited access to health care services are well known risk factors for developing ARF8 . Out of the Jones criteria for the diagnosis of rheumatic fever (major criteria – polyarthritis, carditis, chorea, erythema marginatum, subcutaneous nodules and minor criteria – fever, arthralgia, previous ARF or RHD, acute phase reactions, prolonged PR interval), polyarthritis occurs in 50-70 percent of patients, being the most common and also the least specifi c manifestation in ARF. It usually affects adolescents or young adults, involving large joints unilaterally – knee, elbow, fi st, ankle and rarely the hip. The arthritis migrates, affects one joint for less than two weeks and it responds promptly to nonsteroidal anti-infl ammatory drugs9 . The young woman reported only one ARF episode manifested by knee and hip bilateral arthritis that lasted for approximately two weeks (the medical records of the patient were unavailable). The ARF prevention, both of the initial and the recurrent episodes (primary and secondary prophylaxis), depends on the control of the group A beta-haemolytic streptococcus pharyngitis, accomplished by antibiotherapy10. At least a third of the ARF episodes are caused by subclinical streptococcal infections11, as was the case of our patient who did not present the signs or symptoms of a pharyngitis prior to the development of polyarthritis. Considering that the patients who have suffered an episode of ARF have a predisposition for the recurrence of the event and that streptococcal infections may be asymptomatic, the long-term antibiotherapy is more effi cient for the prevention of a new ARF episode than the treatment of an acute streptococcal pharyngitis. The duration of the prophylaxis depends on the risk of ARF recurrence, however, the optimum antibiotherapy course length is uncertain12. The risk of developing a new ARF episode increases proportionally to the number of previous ARF events and it rises in patients who have a high risk for exposure to streptococcal infections (e.g. close contact with children)6 or suffer from RHD. The risk decreases with age and as the time interval between the ARF episodes widens13. Considering that the young woman had three children aged between 7 and 12 years old and suffered from RHD, the patient had recommendation for secondary prophylaxis of rheumatic fever. After the initial ARF episode, the duration of antibiotherapy is 5 years or until age 21 for patients without carditis, 10 years or up to the age of 21 for those who suffered from carditis but have no residual heart disease and 10 years or until 40 years of age in cases with carditis and RHD. Usually, the longest period is chosen1 . The patient should have been prescribed antibiotherapy for 5 years after the initial ARF episode, which she did not receive. Once the RHD was discovered, the antibiotics were recommended for 10 years, with intramuscular benzathine penicillin G every four weeks as fi rst-line treatment. The patient was first diagnosed with atrial fi brillation and she was not prescribed antibiotics due to the anticoagulant therapy which represents a contraindication for intramuscular injections. However there are orally administered alternatives – penicillin V, sulphonamides or macrolides1 . For our patient penicillin V 250 mg twice a day was recommended at discharge. Rheumatic mitral stenosis is associated with the gradual reduction of the valve area which normally ranges between 4 and 6 cm2 . Usually dyspnoea appears when the mitral area is less than 2.5 cm2 and for surface values lower than 1.5 cm2 (at least moderate stenosis) symptoms at rest may occur – in our case, the valve area estimated by planimetry was 1.1 cm2 14. For better describing the degree of diminution of the mitral valve opening, other echographyc parameters are taken into account – the mean transvalvular pressure gradient and the estimated pulmonary arterial systolic pressure (6 mmHg and 31 mmHg respectively in this case). After the echocardiography, the patient was diagnosed with moderate mitral stenosis (valve area between 1 and 1.5 cm2 , and also mean pressure gradient between 5 and 10 mmHg and PASP between 30 and 50 mmHg15. Manifestations of mitral stenosis include exertional dyspnoea and a decline in exercise tolerance. These symptoms are dependent on the degree of reduction of the valvular area that affects the pressures in the left atrium and the pulmonary circulation, the vascular resistance and the cardiac output. Any situation that leads to the augmentation of the cardiac output, of the transmitral fl ow or induces tachycardia reducing the diastolic fi lling time of the left ventricle, may increase the transvalvular pressure gradient prompting the surfacing of symptoms (e.g. exercise, atrial fi brillation, pregnancy). Atrial fi brillation is frequent in patients with mitral stenosis (due to the increase in atrial pressure that results in the enlargement of the left atrium) and it can be the fi rst signal of the presence of the valve lesion. Infl ammation in the left atrium (demonstrated by sustained increased values of the C-reactive protein for years after the initial ARF episode) enhances the probability of developing this dysrhythmia1 . The prevalence of atrial fibrillation in patients with RHD is 50 percent. When the lesion of the tricuspid valve is also present the prevalence increases to 70 percent16. The fi rst symptoms of the patient were palpitations accompanied by dyspnoea, probably due to the paroxysmal atrial fi brillation. Later, the patient experienced exertional dyspnoea. Chest pain rarely occurs in mitral stenosis, it can be caused by ischemic heart disease or by a coronary embolism, the latter having a very low incidence1 . There was a small probability of signifi cant coronary artery disease, there were no ECG changes evocative of ischemia or alteration in ventricular contraction when echocardiography was performed. The chest pains experienced by the patient can be explained by the sudden increase in the pulmonary pressure or as a functional angina pectoris that occur during tachycardia as a result of atrial fi brillation or exercise. Although ARF equally affects both sexes, two thirds of the patients with RHD are women1 . During pregnancy many haemodynamic changes occur which can lead to cardiac decompensation and signifi cant fetomaternal morbidity and mortality. The rise in the cardiac load leads to development of symptoms such as dyspnoea, palpitations, fatigue, which is why the valve disease is frequently diagnosed during pregnancy17. The risk of cardiac decompensation is infl uenced by the valve lesion severity – heart failure is seen more often in pregnant women who have moderate or severe mitral stenosis, even in patients who were asymptomatic prior to the pregnancy18. In this case, the patient did not present heart failure manifestations during any of the pregnancies (the last child was born approximately 6 years before the rheumatic mitral disease diagnosis was established – at that time the mitral stenosis was probably mild). Also, heart failure manifestations can mimic pregnancy symptoms17. The natriuretic peptides are cardiac hormones with a role in the hydroelectrolitic balance19. BNP is released into the bloodstream in response to excessive stretching of the myocytes during the diastole in diseases with volume or pressure overload of the left ventricle20. BNP and its precursor (NT-proBNP), are well known markers for heart failure20 and signifi cant rises of their plasma levels are detected especially in cardiac decompensation19. BNP levels can be increased in patients with valvulopathies, especially in those with severe lesions (in valve disease with normal LVEF the compensatory ventricular remodelling is not usually associated with high levels of BNP). The atrial expression of the mRNA for BNP is twice as large as the ventricular one and the levels of BNP correlate with the atrial dimensions. However, the quantity of BNP released by the ventricles is more important due to the larger ventricular myocyte mass21. The BNP levels of our patient were normal despite the signifi cant mitral disease – the patient did not present signs or symptoms of decompensated heart failure at the time of the test, the mitral regurgitation (volume overload for the left ventricle) was mild, the atria were enlarged but the systolic ventricular function was preserved. The pharmacological treatment in mitral disease includes diuretics, long acting nitrates for the dyspnoea; beta blockers or calcium channel blockers for the negative chronotropic effect that leads to an increase in the duration of the diastole and better exercise tollerance; oral anticoagulant treatment with coumarin derivatives in a dose adjusted to maintain a target INR of 2.5 – range from 2 to 3 (for paroxysmal or permanent atrial fi brillation; in patients with sinus rhythm the anticoagulant therapy is recommended only when there is history of embolic accidents or when a thrombus is found in the left atrium)22. Our patient was prescribed a coumarin derivative anticoagulant, a beta blocker and a loop diuretic and the treatment alleviated the symptoms. Mitral stenosis induces a hypercoagulability state both locally (in the left atrium) and systemically. The proposed mechanism for the procoagulant state is fl ow obstruction through the mitral valve that leads to an increase in pressure in the left atrium, blood stasis, followed by platelet activation and activation of the coagulation cascade. Tachycardia or the presence of atrial fi brillation further augments the atrial pressure and the rate of activation of coagulation. The stroke risk in patients with atrial fi brillation associated with mitral stenosis and in those with non-valvular atrial fi brillation is 17 and 5 times higher respectively in comparison with a control group. For the atrial fi brillation associated to a valvulopathy the only approved anticoagulant therapy is with vitamin K antagonists and the patient had been prescribed acenocoumarol. Furthermore, rigorous ventricular rate control (with a beta blocker in this case) reduces the risk of atrial thrombus development23. REMEDY was a prospective study of over 3300 cases of RHD which concluded that in endemic countries the rate of contraceptive methods use is extremely low and that more than 20% of the pregnant women with mitral disease were being treated with coumarin derivatives anticoagulants7 . Vitamin K antagonists have teratogenic effects in the fi rst trimester and increase the fetal bleeding risk in the fi nal two weeks of pregnancy24. The patient was at the reproductive age and thus needed a contraceptive method during anticoagulant therapy. No contraception method is infallible. The natural birth control methods – periodic abstinence, coitus interruptus, lactational amenorrhea – are the least effective, with a failure rate between 25 and 55 percent. On the opposite side is surgical sterilization which is effective in 99.5 percent of the cases for the ligation of the fallopian tubes and 99.85% for vasectomy, but the couple has to understand the irreversible nature of the procedure (the method is ideal for monogamous couples who do not want any or more children and in situations where pregnancy would be life threatening – e.g. severe heart disease). Other birth control methods are chemical and mechanical barrier methods sometimes used in association (male and female condoms, diaphragm, cervical cap, spermicidal substances), and intrauterine devices (IUD – usually recommended for monogamous women with a low risk of sexual transmitted diseases or for those with contraindication for hormonal contraceptives). Hormoal methods of birth control include the combined pill (estrogen and progesterone) and the progesterone-only pill25. The patient could use barrier, IUD or she could undergo sterilization as heart disease is among the relative contraindications to hormonal contraception26. Long-term use of amiodarone can induce a series of side effects involving all organ systems. Although most of these side effects are mild and do not occur when the daily dose is lower than 400 mg, some may be fatal. Pulmonary complications (interstitial pneumonitis, pulmonary fi brosis) are the most serious, with an incidence ranging between 0.5 and 10 percent. Thyroid disorders (both hyperthyroidism and hypothyroidism) occur in 2 to 24 percent of the patients. The eye is the most frequently affected organ. Liver injuries may occur, but usually the increase in transaminases and alkaline phosphatase levels are asymptomatic and reversible when the amiodarone dose is lowered or the treatment is ceased. Thus, patients treated with amiodarone should undergo a check-up that includes a chest x-ray, thyroid and liver function tests, ophthalmological exam both at the start of the therapy and at regular intervals afterward. A close INR monitoring is necessary at the beginning and during the antiarrhythmic treatment due to the interactions between amiodarone and vitamin K antagonists (amiodarone potentiates the anticoagulant effect as early as 3 days after the debut of the antiarrhythmic therapy). The patient’s chest x-ray, thyroid and liver functions were normal and she had a therapeutic INR at the moment of the admittance. Ocular adverse effects, usually in the form of corneal microdeposits (in 98 percent of patients after a cumulative dose of 9 g or one month of treatment), but lens opacities, optic nerve neuropathy and colour vision impairment may develop. Usually, the ocular side effects of amiodarone are asymptomatic. A small number of patients complain of photophobia, blurred vision and halos; however, the cessation of the treatment is rarely necessary27. Our patient who had received more than 9 g of amiodarone in the course of a year, presented none of these symptoms. Amblyopia is a unilateral or bilateral decrease of visual acuity, caused by deprivation of form sense of vision during the critical period of nervous system development, resulting in an abnormal binocular interaction28. Amblyopia is induced by anisometropia, strabismus and rarely by deprivation of visual stimuli (corneal ulcer or scarring, congenital cataract, palpebral ptosis, glaucoma, ocular injuries or surgery) – the last two mechanisms were not identifi ed in this case. The patient was diagnosed with anisometropic hypermetropia and astigmatism. There is proof that astigmatism plays a role in the development of amblyopia29, and the patient presents this disease in the amblyopic eye. It is worth mentioning that although the patient had had impaired vision for decades, the fi rst ophthalmological examination of her life was performed in our clinic. It is highly likely that had the amblyopia been treated during her childhood, the patient’s eyesight would have been better. There is one study that observed the effect of occlusion of the better eye with vision training for 2-4 hours daily in adolescents and young adults; a signifi cant eyesight improvement was reported. The patient could benefi t from this treatment in an ophthalmological clinic28. There is no evidence that ARF leads to ocular complications30, thus the patient’s visual impairment is not caused by this disease. When a lesion of a cardiac valve is present, the risk of developing infectious endocarditis increases (IE)31, however, in RHD this complication occurs in less than 20 percent of cases1 . IE is more frequently encountered when the mitral stenosis is mild and the valves are thick, with fi brous degeneration. The incidence decreases as the valve becomes calcifi ed and rigid31. The patient has noncalcifi ed mobile leafl ets and is thus at risk of developing this complication, but according to the current guidelines antibiotic prophylaxis for IE is not necessary in this case32. Percutaneous balloon valvuloplasty involves inserting one ore more balloons fi lled with fl uid in the left ventricle transseptally of retrogradely. After entering the mitral valve, the balloons are infl ated, fracture the fused commissures, thus widening the valve opening and increasing the mobility of the leafl ets1 . It is the fi rst-line treatment in rheumatic mitral stenosis in patients without contraindications to the intervention – MVA greater than 1.5 cm2 , severe calcifi cations in one or both commissures, the lack of commissural fusion, severe associated lesions in the aortic valve or the presence of tricuspid disease (severe stenosis and regurgitation), coronary heart disease that requires surgical revascularization, left atrial thrombosis (including appendage thrombosis), more than mild severity of the mitral regurgitation. In these cases, surgery is recommended in symptomatic patients with a MVA less than 1.5 cm2 22. The echographic assessment of the mitral valve morphology plays a key role in choosing the best treatment option. Leafl ets’ mobility and fl exibility, their thickening and calcifi cation degree, commissural and subvalvular apparatus fusion can be assessed with echocardiography. These traits are included in scores that help determine the suitability of mitral balloon valvuloplasty and predict its outcome. The Wilkins score evaluates the severity and extent of leafl et calci- fi cation and thickening, the valve opening restriction, the subvalvular apparatus involvement. Assessment of commissural calcium is a predictor of achieving an MVA greater than 1.5 cm2 after the valvuloplasty without creating a signifi cant mitral regurgitation (each commissural half is given a score of 1 if high-intensity bright echoes are detected; the maximum score is 4)33. The Cormier score divides the patients in three groups: group 1 – pliable noncalcifi ed anterior mitral leafl et and mild subvalvular disease (thin chordae more than 10 mm long); group 2 – pliable noncalci- fi ed anterior mitral leafl et but severe subvalvular involvement (thickened chordae less than 10 mm long); group 3 – calcifi cation of the mitral valve of any extent whatever the state of the subvalvular apparatus34. A score based on real-time 3-dimensional transthoracic echocardiography (RT-TT3DE) was developed, having a better predictive value for the success of the valvuloplasty compared with the other scores. The commissures and the leafl et scallops are better evaluated with 3D echocardiography, revealing the irregularly situated morphological anomalies that are encountered in rheumatic mitral stenosis35. All of these semi-quantitative scores are complementary to each other and the variable reproducibility is one of their limits. New quantitative parameters that could be included in the assessment of the valvular morphology are the maximal excursion of the leafl ets from the annulus in diastole and the asymmetry in commissural remodelling36. The patient has no unfavourable characteristics or contraindications for balloon mitral valvuloplasty (a Wilkins score greater than 8, a Cormier score of 3, a very limited valve area, severe tricuspid regurgitation1 ). When considering the clinical characteristics associated with a poor prognosis (old age, history of commissurotomy, NYHA class IV heart failure, atrial fi brillation, pulmonary hypertension1 ), only atrial fi brillation is present in this case. An immediate favourable result of interventional therapy is obtaining a MVA greater than 1.5 cm2 (or 50 percent greater than the initial value), with mitral regurgitation that is mild at most1 . An increase from 1 cm2 to 2 cm2 in the MVA is reported in most of the studies concerning balloon mitral valvuloplasty. Although the obtained MVA is smaller than the normal opening of the mitral valve, the increase is sufficient to produce a substantial hemodynamic improvement: a decrease in left atrial pressure, transmitral gradient, pulmonary pressure, and a rise in the cardiac output37. In this case, an increase of over 50 percent from the initial value in the MVA, a decrease in the mean transmitral gradient and PASP (from 6 to 4 mmHg and from 31 to17 mmHg respectively) were noted. Also, a signifi cant amelioration of the symptoms was acknowledged. Approximately 10 percent of patients require reintervention 7 years after the fi rst mitral balloon valvuloplasty. The intervention can be repeated as long as valvular morphology remains favourable and none of the contraindications for it have arisen. The result is generally poorer when the intervention is repeated, as at this time calcification, fibrosis and valvular deformities are usually more notable than the initial anomalies38.
CONCLUSION
The most notable characteristics of this case are the absence of symptoms or maternofoetal complications during all three pregnancies, the initial ARF episode without a symptomatic pharyngotonssilitis to precede it, the involvement of the coxofemoral articulations during the polyarthritis. The lack of antibiotic prophylaxis in a patient at risk for an ARF recurrence considering the presence of RHD and the close contact with children is also worth mentioning. The young woman receives anticoagulant therapy that requires the use of birth control, however hormonal methods (the most frequently used in the general population) have a relative contraindication in this case – for our patient barrier methods, IUD or surgical sterilization are more viable options. The valvular lesion must be closely monitored, as someday invasive procedures may be necessary once again – another percutaneous balloon valvuloplasty or mitral valve replacement surgery.
Conflict of interest: none declared.
References
1. C Ginghina, A Calin. Mic tratat de cardiologie. Bucuresti : Editura Academiei Romane, 2010.
2. D. L. Mann, D P. Zipes, P. Libby, R. O. Bonow, E. Braunwald. Braunwald’s Heart Disease: a Textbook of Cardiovascular Medicine Tenth Edition. s.l. : Elsevier, Saunders, 2015.
3. Carapetis JR, Steer AC, Mulholland EK, Weber M. The global burden of group A streptococcal diseases. s.l. : The Lancet. Infectious diseases, 2005.
4. Marijon E, Mirabel M, Celermajer DS, Jouven X. Rheumatic heart disease. s.l. : Lancet, 2012.
5. Marcus RH, Sareli P, Pocock WA, Barlow JB. The spectrum of severe rheumatic mitral valve disease in a developing country. Correlations among clinical presentation, surgical pathologic fi ndings, and hemodynamic sequelae. s.l. : Annals of Internal Medicine, 1994.
6. Bland EF, Duckett Jones T. Rheumatic fever and rheumatic heart disease; a twenty year report on 1000 patients followed since childhood. s.l. : Circulation, 1951. Vol. 4.
7. Zühlke L, Engel ME, Karthikeyan G, Rangarajan S, Mackie P, Cupido B, Mauff K, Islam S, Joachim A, Daniels R, Francis V, Ogendo S, Gitura B, Mondo C, Okello E, Lwabi. Characteristics, complications, and gaps in evidence-based interventions in rheumatic heart disease: the Global Rheumatic Heart Disease Registry. 2015.
8. Essop MR, Peters F. Contemporary issues in rheumatic fever and chronic rheumatic heart disease. s.l. : Circulation, 2014.
9. Eroglu, Ayşe Güler. Update on diagnosis of acute rheumatic fever: 2015 Jones criteria. s.l. : Turk Pediatri Ars, 22016.
10. GH, Stollerman. Rheumatic fever. London : Lancet, 1997.
11. AS, Dajani. Current status of nonsuppurative complications of group A streptococci. s.l. : The Pediatric infectious disease journal, 1991.
12. Gerber MA, Baltimore RS, Eaton CB, Gewitz M,. Prevention of rheumatic fever and diagnosis and treatment of acute Streptococcal pharyngitis: a scientifi c statement from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee of the Council on Cardiovascular Disease. s.l.: Circulation, 2009.
13. Majeed HA, Yousof AM, Khuffash FA, Yusuf AR. The natural history of acute rheumatic fever in Kuwait: a prospective six year follow-up report. s.l. : Journal of Chronic Diseases, 1986.
14. Hugenltz PG, Ryan TJ, Stein SW, Abelmann WH. The spectrum of pure mitral stenosis. Hemodynamic studies in relation to clinical disability. s.l. : The American Jounal of Cardiology, 1962. Vol. 10.
15. Baumgartner H, Hung J, Bermejo J. Echocardiographic assessment of valve stenosis: EAE/ASE. s.l. : European Journal of Echocardiography, 2009.
16. Diker E, Aydogdu S, Ozdemir M,. Prevalence and predictors of atrial fi brillation in rheumatic valvular heart disease. s.l. : American Journal of Cardiology, 1996.
17. Nazia Ahmed, Hafeeza Kausar, Lubna Ali. Fetomaternal outcome of pregnancy with Mitral stenosis. s.l. : Pakistan journal of medical sciences, 2015.
18. Regitz-Zagrosek V, Blomstrom Lundqvist C, Borghi C. ESC Guidelines on the management of cardiovascular diseases during pregnancy. s.l. : European Heart Journal, 2011.
19. Langenickel T, Pagel I, Höhnel K, Dietz R, Willenbrock R. Differential regulation of cardiac ANP and BNP mRNA in different stages of experimental heart failure. s.l.: American journal of physiology. Heart and circulatory physiology, 2000.
20. Bergler-Klein J, Gyöngyösi M, Maurer G. The role of biomarkers in valvular heart disease: focus on natriuretic peptides. s.l. : The Canadian journal of cardiology, 2014.
21. Sharma V, Stewart RA, Lee M. Plasma brain natriuretic peptide concentrations in patients with valvular heart disease. s.l. : Open Heart, 2016.
22. Alec Vahanian, Ottavio Alfi eri, Felicita Andreotti, Manuel J. Antunes, Gonzalo Barón-Esquivias, Helmut Baumgartner, Michael Andrew Borger, Thierry P. Carrel , Michele DeBonis. Guidelines on the management of valvular heart. s.l. : European Heart Journal (2012), 2012.
23. Yusuf J, Goyal M, Mukhopadhyay S. Effect of heart rate control on coagulation status in patients of rheumatic mitral stenosis with atrial fibrillation–A pilot study. s.l. : Indian Heart Journal, 2015.
24. Dobrescu D, Negres S, Dobrescu L. Memomed 2016 – Memorator de farmacologie alopata. Bucuresti : Editura Universitara, 2016.
25. Tamara Callahan, Aaron B. Caughey. Blueprints – Obstetrics & Gynecology. s.l. : Lippincott Williams & Wilkins, 2013.
26. Errol R. Norwitz, John O. Schorge. Obstetrics and Gynecology at a Glance. s.l. : Wiley-Blackwell, 2013.
27. Jafari-Fesharaki M, Scheinman M. Adverse Effects of Amiodarone. s.l. : Pace, 1998.
28. T, Khan. Is There a Critical Period for Amblyopia Therapy? Results of a Study on Older Anisometropic Amblyopes. s.l.: Journal of clinical and diagnostic research, 2015.
29. EM, Harvey. Development and treatment of astigmatism-related amblyopia. s.l. : Optometry and vision science, 2009.
30. Lenoch F, Kralik V, Bartos J. “Rheumatic” Iritis and Iridocyclitis. s.l.: Annals of the rheumatic diseases, 1959
31. Catherine M Otto, William H Gaasch, Susan B Yeon. Clinical manifestations and diagnosis of mitral stenosis. 2015.
32. Gilbert Habib, Patrizio Lancellotti, Manuel J. Antunes. 2015 ESC Guideline for the management of infective endocarditis. s.l.: European Heart Journal, 2015.
33. Wunderlich NC, Beigel R, Siegel RJ. Management of Mitral Stenosis Using 2D and 3D Echo-Doppler Imaging. s.l.: JACC. Cardiovascular imaging, 2013.
34. Ginghina C, Popescu BA, Jurcut R. Esentialul in ecocardiografi e. Bucuresti: Editura Medicala Antaeus, 2013.
35. NC, Nanda. Comprehensive Textbook of Echocardiography. s.l.: Jaypee Brothers Medical Publishers, 2014.
36. Nunes MC, Tan TC, Elmariah S, Lago R. The Echo Score Revisite. 2014: Circulation.
37. Carroll JD, Feldman T. Percutaneous mitral balloon valvotomy and the new demographics of mitral stenosis. s.l. : Journal of the American Medical Association, 1993.
38. Ben Farhat M, Ayari M, Maatouk F, Betbout F, Gamra H, Jarra M, Tiss M, Hammami S, Thaalbi R, Addad F. Percutaneous balloon versus surgical closed and open mitral commissurotomy: seven-year follow-up results of a randomized trial. s.l. : Circulation. Vol. 1998.
 This work is licensed under a
This work is licensed under a