Anca D. Mateescu1,2, Francesco Antonini-Canterin2, Gian Luigi Nicolosi3
1 “Prof. Dr. C.C. Iliescu“ Institute of Cardiovascular Diseases, Bucharest, Romania
2 Cardiologia Preventiva e Riabilitativa, ARC, Azienda Ospedaliera S. Maria degli Angeli, Pordenone, Italy
3 Cardiologia, ARC, Azienda Ospedaliera S. Maria degli Angeli, Pordenone, Italy
Contact address:
Anca D. Mateescu, MD
„Prof. Dr. C.C. Iliescu” Institute of Cardiovascular Diseases, Bucharest
Sos. Fundeni 258, sector 2, 022328
Bucharest, Romania
Phone/Fax: +40213175227
E-mail: ancad.mateescu@gmail.com
Abstract: Pulmonary embolism is a multifactorial disease that carries a high risk of mortality. Although venous thromboembolism may occur in patients without any identifiable predisposing risk factors, one or more of them are usually identified. It has been reported that approximately 5% of patients with acute pulmonary embolism present with right heart thrombus which occasionally straddles the interatrial septum, posing a threat to the pulmonary arteries and systemic circulation. Despite the high prevalence of patent foramen ovale in the general population, paradoxical embolism is a rare event. The treatment of pulmonary embolism in patients with impending systemic embolism remains controversial. According to a statement from the American Heart Association, surgical thrombectomy in patients with an intracardiac thrombus might reduce the incidence of stroke, whereas thrombolysis might be associated with higher mortality. The ESC guidelines on the diagnosis and management of acute pulmonary embolism concludes that both thrombolysis and embolectomy are effective whereas anticoagulation alone appears less effective.
Keywords: impending paradoxical embolism, abortion, pulmonary embolectomy
Rezumat: Trombembolismul pulmonar este o boală multifactorială cu risc crescut de mortalitate. Deși trombembolismul venos poate apărea fără factori predispozanți decelabili, de obicei unul sau mai mulți factori pot fi identificați. Aproximativ 5% dintre emboliile pulmonare prezintă trombi intracardiaci drepți care, ocazional, pot traversa septul interatrial, conferind un risc crescut de embolie sistemică. În ciuda prevalenței crescute a foramenului ovale patent, embolia paradoxală este rar întâlnită. Tratamentul trombembolismului pulmonar la pacienții cu iminență de embolie sistemică este controversat. Conform ghidurilor americane, trombectomia chirurgicală poate reduce incidența accidentului vascular în cazul trombilor intracardiaci, în timp ce tromboliza se asociază cu o rată a mortalității mai mare. Ghidul european de diagnostic și management al emboliei pulmonare conchide că atât tromboliza cât și embolectomia sunt eficiente în timp ce tratamentul anticoagulant este mai puțin eficient.
Cuvinte cheie: embolie paradoxală, avort, embolectomie pulmonară
INTRODUCTION
Pulmonary embolism (PE) is a multifactorial disease that carries a high risk of mortality. Although PE can occur in patients without any identifiable predisposing risk factors, one or more of them are usually identified. Predisposing factors for venous thromboembolism (VTE) include surgery, major trauma, malignancy, oral contraceptive therapy, hormone replacement therapy, thrombophilia, pregnancy, postpartum, obesity, smoking, bed rest for more than 3 days, immobility due to sitting (car or air travel)1. Despite the high prevalence of patent foramen ovale (PFO) in the general population, (estimated at 26%), paradoxical embolism is a rare event1. It requires the following diagnostic criteria, originally proposed by Johnson2: (1) presence of deep venous thrombosis (DVT) and/or PE; (2) abnormal communication between the right and left circulation; (3) clinical, angiographic, or pathologic evidence of systemic embolism; and (4) presence of a favorable pressure gradient promoting right-to-left shunting.
CASE REPORT
A 39-year-old woman was admitted to the gynecological department for spontaneous abortion in the 18th week of pregnancy due to premature amniotic membrane rupture. After one week, the patient was complaining of shortness of breath, palpitations and chest pain, which she considered to be secondary to the mechanical constriction used for stopping the lactation process. The patient is a former smoker with a history of taking oral contraceptives (for 10 years), in vitro fertilisation, recent prolonged air travel (for 12 hours) and family history of DVT. Physical examination revealed tachypnea, regular heart rate of 82 beats per minute, blood pressure of 105/70 mmHg and pulse oximeter oxygen saturation of 98% on air. Cardiac auscultation revealed a systolic murmur at the left lower sternal border and a splitting of the second heart sound in the pulmonary area. The patient also presented pain, swelling and tenderness in her right leg. There were no additional abnormalities on physical examination.
The ECG showed a sinus rhythm at 82 bpm and non-specific ST-T changes (Figure 1). Blood analysis was unremarkable except for a significant increase D-dimer assay, inflammatory syndrome and mild anemia (Hb 9.4 g/dl). Chest X-ray showed right pulmonary hilum and inferior arch enlargement, right lung transparent areas, and elevated hemidiaphragm. Transthoracic echocardiography revealed mild right chambers dilation, right ventricular dysfunction, and paradoxical interventricular septal wall motion (consistent with elevated right heart pressures). The assessment of pulmonary artery systolic pressure was carried out by measuring the peak tricuspid regurgitation velocity—the estimated value was 45 mm Hg. The left ventricular function was normal. From the left parasternal short axis we observed a mobile mass attached to the pulmonary valve which was oscillating between the right ventricular outflow tract and the pulmonary artery, compatible with a thrombo-embolus (Figure 2). Moreover, the apical four chambers view revealed a large serpentine and a mobile thrombus in right atrium extending through a PFO into the left atrium with the extremities inclavated into the atrioventricular valves (Figure 3).
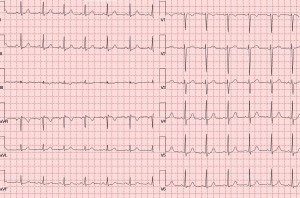
Figure 1. Electrocardiogram revealed synus rithm at 82 bpm, QRS axis at +37°, and non-specific ST-T changes.
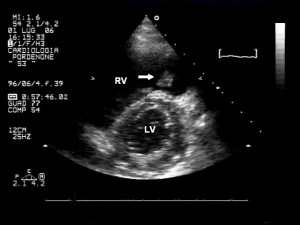
Figure 2. Transthoracic echocardiogram; left parasternal short axis at papillary muscles revealed a mild right ventricle dilation and a diastolic flattening of the interventricular septum which is consistent with elevated right heart pressures. We also observed a mobile mass attached to the pulmonary valve (arrow) which was oscillating between right ventricular outflow tract and pulmonary artery (compatible with a thrombus). RV- right ventricle, LV – left ventricle.
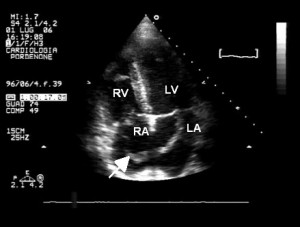
Figure 3. Transthoracic echocardiogram—apical four chambers view. Large serpentine and mobile thrombus (arrow) in right atrium passing through a patent foramen ovale into the left atrium. RV- right ventricle, RA – right atrium, LV- left ventricle, LA – left atrium.
Compression ultrasonography of the lower extremities revealed right femoral and popliteal veins thrombosis (Figure 4). Right pulmonary artery embolism was confirmed by Computed tomography (CT) angiogram (Figure 5).
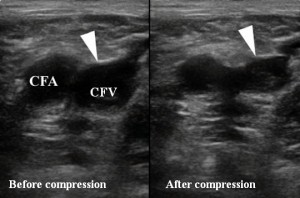
Figure 4. Duplex ultrasonography of the right femoral vein—lack of compressibility suggesting thrombosis. CFA—common femoral artery, CFV—common femoral vein.
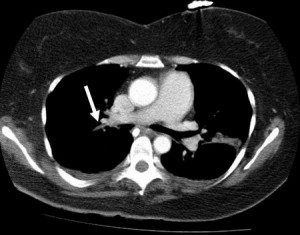
Figure 5. CT angiogram—an intraluminal filling defect which suggests that the right pulmonary artery was occluded (arrow).
The final diagnosis was:
- Acute pulmonary embolism
- Multicavity intracardiac thrombosis (right atrium, left atrium, right ventricular outflow tract)
- Patent foramen ovale
- Acute femoral and popliteal vein thrombosis
- Recent spontaneous abortion
Due to a very high risk of further sistemic embolism with potential disastrous consequences, we initiated endovenous perfusion with unfractionated heparin (IA) and we transferred the patient to a tertiary cardiac centre. The surgeons removed the intracardiac thrombus, performed pulmonary embolectomy and closed the PFO. Histopathologic examination confirmed the diagnosis of thrombus. One day after surgery, due to the persistence of the superficial femoral vein thrombosis, a venous filter was inserted into the inferior vena cava for short term (IIb). Conventional oral anticoagulant therapy with Warfarin was initiated for 6 months. At discharge, the patient was tested for thrombophilia. The extension of the anticoagulant treatment will be evaluated according to the thrombophilia test results. Follow-up to one month after surgery was uncomplicated and the transthoracic echocardiogram showed normal dimensions of right cardiac chambers and no signs of pulmonary arterial hypertension or thrombosis. The trombophilia test results were not available at one month after surgery.
DISCUSSIONS
An impending paradoxical embolus is an uncommon finding in PE patients. The first case was described by Zahn in 18813. If the thrombus is sufficiently large in comparison with the size of PFO, it can be trapped during its passage from the right to the left atrium, determining an impending paradoxical embolism. This carries a high mortality and requires urgent intervention to prevent sistemic embolization. Routine echocardiography in these patients would perhaps increase the incidence of finding a trapped thrombus in a PFO. One study of 174 patients with right intracardiac thrombi reported a 24-hour mortality rate of 11.5%4. More than 90% of these patients already had evidence of PE at the time of presentation and 55% had evidence of systemic embolization. The optimal treatment is controversial in the absence of controlled trials. In the ICOPER registry, thrombolytic treatment was the preferred option, but the 14-day mortality was still high (20.8%)1. An additional advantage of thrombolytic therapy is the simultaneous thrombolysis of cardiac and pulmonary arterial thrombi as well as of deep vein thrombosis, which may be responsible for recurring of embolisms5. Kinney et al. have reported a greater survival rate after pulmonary embolectomy in right heart thrombi than after systemic thrombolysis or anticoagulation6. The ESC guidelines on the diagnosis and management of acute PE concludes that both thrombolysis and embolectomy are effective whereas anticoagulation alone appears less effective1.
Our patient had an increased number of predisposing factors for venous thromboembolism (history of family DVT, oral contraceptive therapy, in vitro fertilization, pregnancy, recent surgery for spontaneous abortion, bed rest more than 3 days, prolonged air travel).
Pregnancy is a predisponsing factor VTE. PE is the most common cause of maternal death in developed countries7. At least 50% of women that develop VTE associate thrombophilia8. Guidelines recommend genetic testing for detection of hypercoagulable states by all pregnant women who have family history of VTE before age of 50 years or complications during pregnancy (one or more miscarriages, fetal death or inadequate growth)7.
Another predisposing factor for VTE is in vitro fertilisation. Henriksson and his colleagues have recently reported that in vitro fertilisation is associated with an increased risk of pulmonary embolism and venous thromboembolism during the first trimester9. Studies recommend developing VTE risk evaluation in women with desire of pregnancy through in vitro fertilisation8,9. Low molecular weight heparin may be used to prevent deep vein thrombosis in risk groups10.
For people traveling on long flights, the risk of deep vein thrombosis or pulmonary embolism is real and dangerous if left unrecognized or untreated11. Although the absolute risk is very low, this threat appears to be about three times higher in travelers and increases with longer trips12. Pregnant women carry a doubled risk and this may increase if the patient associates thrombophilia13. Low molecular weight heparin may be used before the flight to prevent VTE14.
Considering the patients’ family history of DVT, screening for thrombophilia was required. The most common types of congenital thrombophilia are factor V Leiden (a mutation in the F5 gene at position 1691), prothrombin G20210A, a mutation in prothrombin (at position 20210 in the 3’ untranslated region of the gene) and methylenetetrahydrofolate reductase (MTHFR C677T şi A1298C)15. Individuals of C677T are predisposed to mild hyperhomocysteinemia, because they have less active MTHFR available to produce 5-methyltetrahydrofolate, which is used to decrease homocysteine. Studies showed that individuals with high levels of homocysteine are at risk for VTE, but this remains controversial and still under debate15.
The duration of anticoagulant treatment was a difficult decison. The duration of the therapy varies according to the severity and localization of thrombosis, as well as the character of transient and permanent occurrence of conditions that favor VTE. The identification of predisposing factors for VTE development allows an accurate assessment of the need for maintenance treatment. According to ESC guidelines, we have decided that the patient should receive Warfarin for at least 6 months. We also advised the patient to stop using oral contraceptives.
Given the new directions of anticoagulant therapy, we could have taken into account Dabigatran, given the efficacy which has been proved to be similar to Warfarin’s in preventing the recurrence of PE; but this also has a similar risk of bleeding without the need to monitor the INR or adjust the dosage16,17.
Long-term patient follow up is essential, with both clinical and echocardiographic regular assessement because the risk of VTE recurrence and chronic thromboembolic pulmonary hypertension persists even months or years after the initial episode18.
This case has the following peculiarities: patient with first pregnancy at an advanced age and predisposing factors for VTE; multicavity intracardiac thrombosis; associating a PFO that allowed the penetration of thrombus in the left atrium, conferring an increased risk of paradoxical systemic embolism; relatively good clinical condition, discordant with the degree of pulmonary artery affecting; the deception caused by the mechanical breast constriction used to stop lactation, which resulted in patient minimizing her symptoms, thereby delaying diagnosis.
In summary, our patient represents one of the few cases of impending paradoxical embolism reported. With the increasing prevalence of routine transthoracic and transesophageal echocardiograms, this finding may become more common. Any patient with neurological changes complicating cardiovascular events, DVT or PE, or any unexplained arterial embolism should be regarded with a high level of clinical suspicion for paradoxical embolism. Suggestions for the evaluation and treatment of patients with impending paradoxical embolism have been outlined. The benefit of these approaches has not been tested in controlled clinical trials and requires further investigation. It is hoped that the use of transesophageal coupled with increased awareness of the phenomenon of paradoxical embolism will help prevent catastrophic consequences by permitting timely surgical intervention.
Conflict of interests: none declared.
References
1. Torbicki A, Perrier A, Konstantinides S, et al. Guidelines on the diagnosis and management of acute pulmonary embolism. Eur Heart J 2008; 29:2276-2315.
2. Johnson B.I. Paradoxical embolism. J. clin. Path. 195; 4: 316.
3. Zahn FW. Thrombose de plusieurs branches de la veine cave inférieure avec embolies consécutives dans les artères pulmonaire, splénique, rénale et iliaque droite. Rev Med Suisse Romande 1881; 1:227-37.
4. Chartier L, Béra J, Delomez M, et al. Free-floating thrombi in the right heart: diagnosis, management, and prognostic indexes in 38 consecutive patients. Circulation 1999;99:2779-83.
5. Zerio C, Canterin FA, Pavan D, Nicolosi GL. Spontaneous closure of a patent foramen ovale and disappearance of impending paradoxical embolism after fibrinolytic therapy in the course of massive pulmonary embolism. Am J Cardiol. 1995; 76:422-4.
6. Kinney EL, Wright RJ. Efficacy of treatment of patients with echocardiographically detected right sided heart thrombi: a meta-analysis. Am Heart J 1989;118:568-73.
7. Leung AN, Bull TM, Jaeschke R, et al. Committee on Pulmonary Embolism in Pregnancy. American Thoracic Society documents: an official American Thoracic Society/Society of Thoracic Radiology Clinical Practice Guideline–Evaluation of Suspected Pulmonary Embolism in Pregnancy. Radiology 2012; 262:635-46.
8. Marik PE, Plante LA. Venous thromboembolic disease and pregnancy. N Engl J Med 2008; 359:2025-2033.
9. Henriksson P, Westerlund E, Wallén H, et al. Incidence of pulmonary and venous thromboembolism in pregnancies after in vitro fertilisation: cross sectional study. BMJ. 2013; 346:e8632.
10. Cutts BA, Dasgupta D, Hunt BJ. New directions in the diagnosis and treatment of pulmonary embolism in pregnancy. Am J Obstet Gynecol. 2013; 208:102-8.
11. Santin A, Renaud B. Symptomatic travel associated pulmonary embolism: high severity does not imply poor long term prognosis. Eur Heart J. 2009; 30:133-4.
12. Bartholomew JR, Schaffer JL, McCormick GF. Air travel and venous thromboembolism: minimizing the risk. Cleve Clin J Med. 2011; 78: 111-20.
13. Martinelli I, Taioli E, Battaglioli T. Risk of Venous Thromboembolism After Air Travel Interaction With Thrombophilia and Oral Contraceptives. Arch Intern Med. 2003; 163:2771-2774 .
14. Sugerman HJ, Eklöf BG, Toff WD, Burke AE, Livingston EH. Air travel-related deep vein thrombosis and pulmonary embolism. JAMA. 2012; 308:2531.
15. Köktürk N, Kanbay A, Aydoğdu M. Hyperhomocysteinemia Prevalence Among Patients With Venous Thromboembolism. Clin Appl Thromb Hemost. 2011; 17:487-93.
16. Schulman S, Reilly PA. Dabigatran etexilate: future directions in anticoagulant treatment. Clin App Thromb Hemost. 2009; 15: 32S-41S.
17. Ferrer E. Dabigatran etexilate in venous thromboembolism. Drugs Today 2009; 45:715-24.
18. Ginghină C. Mic tratat de cardiologie. Editura Academiei, 2010: 655-668.
 This work is licensed under a
This work is licensed under a