Andreea Pârv1, Ovidiu Anchidin1, Camelia Ober1, Dan Bindea2, Mihai Ober3, Caius Romulus Duncea4
1 “Niculae Stancioiu” Heart Institute, Department of Cardiology, “Iuliu Hatieganu”, University of Medicine and Pharmacy, Cluj-Napoca, Romania
2 “Niculae Stancioiu” Heart Institute, Department of Cardiovascular Surgery, “Iuliu Hatieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania
3 Medical Clinic I, Department of Interventional Cardiology, Cluj-Napoca, Romania
4 “Iuliu Hatieganu” University of Medicine and Pharmacy, Cluj-Napoca, Romania
Contact address:
Andreea Pârv, 7 Eugen Ionesco Street, Cluj-Napoca, Romania.
E-mail: andreea.parv@yahoo.com
Abstract: Objectives – The study aims to establish cut-off values for two transthoracic Doppler parameters (the peak diastolic/ systolic velocity ratio- DSVR and the diastolic fraction- DF) which would allow one to differentiate between patent and dysfunctional left internal mammary artery (LIMA) graft. Methods – It was a prospective study which consisted of 30 consecutive patients with at least one LIMA graft, and acute coronary syndrome, investigated by transthoracic Doppler echocardiography (TTE) and coronary angiography. We measured the DSVR and the DF (ratio of the diastolic to total time velocity integral) from the left parasternal window; we have also noted the presence of the systolic reversal of flow (SRF).
Results – A DSVR < 0.32 and a DF < 49% identified dysfunctional LIMA grafts with 100% specificity and 50% and 75% sensitivity respectively. A DSVR ≥ 0.5 and a DF ≥ 51% identified patent LIMA grafts with 88% specificity and 75% sensitivity. For a DSVR between 0.32 and 0.5 and a DF between 49% and 51%, an alternative method to discriminate between patent and dysfunctional LIMA graft would be needed. The presence of SRF usually indicates LIMA-LAD competitive flow and a dysfunctional graft. Conclusion – The TTE of the LIMA graft is a noninvasive and accurate method for discriminating between patent and dysfunctional LIMA graft.
Keywords: left internal mammary artery graft, transthoracic Doppler echocardiography, diastolic fraction, peak diastolic to systolic velocity ratio
INTRODUCTION
In recent years, the left internal mammary artery (LIMA) has become the conduit of choice for the left anterior interventricular artery (LAD) in coronary artery bypass graft (CABG) surgery, being associated with long-term patency1. Until a couple of years ago, its assessment required invasive investigation, usually by conventional angiography, which is still considered the golden standard2 for detecting graft stenosis3. However, angiography is limiting because of its invasive nature, its cost and availability. The routine tests used to assess myocardial ischemia are difficult to interpret in patients with previous CABG, which is why an alternative method to analyze grafts is needed. The flow in LIMA has been investigated by transthoracic Doppler echocardiography (TTE) since the early 1990s4. TTE is a method that is noninvasive, easy to use, reproducible, inexpensive5, without complications and applicable to all patients, including those with a contraindication to contrast medium injections. It can be used both in the immediate and late postoperative period in patients with a history of CABG and recurrent myocardial ischemia. Performing TTE of the LIMA graft requires the operators to carry out training and results interpretation, especially as there is not a singular parameter that can accurately confirm or refute a dysfunctional or patent LIMA.
This prospective study aims to establish, by means of two parameters (the ratio of peak diastolic to systolic velocities and the diastolic fraction), the role of TTE in identifying patent or dysfunctional LIMA grafts, which will require control angiography.
METHODS
Study population
It was a prospective study, which comprised 30 patients with previous CABG, with at least one LIMA graft, presenting with acute coronary syndromes (ACS): unstable angina (UA), acute non-ST and ST-elevation myocardial infarction (NSTEMI and STEMI), between January 2011 and June 2011, at the Heart Institute in Cluj-Napoca. We chose patients with ACS because they all had a coronary angiography on the same day as the echocardiographic investigation of the LIMA graft. We performed TTE for the LIMA graft in all patients before performing a coronary angiography. The study protocol was approved by the hospital ethics committee and all patients gave written consent to both examinations.
Transthoracic Doppler echocardiography of the LIMA graft
The ultrasound examination was performed using a Vivid S6, GE, Healthcare ultrasound unit, equipped with a 7.5 MHz linear transducer. Transthoracic visualization of the LIMA was achieved via the 3rd to the 5th parasternal intercostals approach, as was previously described5,6,7,8,9, with the patients laying in a relaxed supine position. We identified the main body of the LIMA graft via two-dimensional color flow mapping along the 3rd up to the 5th intercostal space. Using the pulsed Doppler method and 2-3 mm sampling volume located within the vessel lumen we obtained intraluminal flow signal (Figure 1). Angle correction was applied for the velocity measurements. The flow pattern of a coronary graft is usually biphasic with a diastolic predominance (similar to a native coronary artery) (Figure 1). In cases of graft dysfunction, the diastolic component is reduced or disappears5.
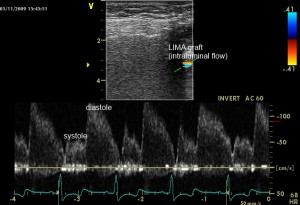
Figure 1. Normal aspect of the LIMA graft by TTE, as seen in color Doppler (up) and pulsed- wave Doppler (down) from the 3 or 4 left intercostals space.
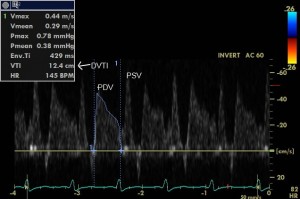
We measured the following parameters: the peak systolic (PSV- cm/s) and diastolic (PDV- cm/s) velocities, the systolic and diastolic velocity time integrals (SVTI- cm and DVTI- cm) (Figure 2). Offline we calculated peak diastolic to peak systolic velocity ratio DSVR (PDV/PSV) and the diastolic fraction (DF-%) as follows: . The DF expresses how much the diastolic flow represents in relation to the total (systolic plus diastolic) flow9. Diastolic and systolic peak blood velocities and their velocity-time integrals and ratios were measured by tracing out the contour of the Doppler velocity curve. The baseline values for each parameter were obtained by averaging measurements from five to seven consecutive cardiac cycles. We also noted the presence of systolic reversal flow, a particular aspect of the LIMA graft flow, usually indicating a dysfunctional graft10,11 due to competitive flow with the native LAD. This finding, which has been reported by other authors7,10,11, could be attributed to a delay in the pressure wave of the LIMA grafts12 under competitive flow conditions from the native LAD.
The flow parameters of the TTE were measured blind to clinical and angiographic results.
Coronary angiography
Cardiac catheterization was performed on the same day, after the Doppler study, by expert angiographers unaware of the echocardiographic findings. Cardiac catheterization was performed by percutaneous right femoral or radial approach, as previously described13.
Angiographic results for the LIMA graft were noted as follows: patent (stenosis <50%), with competitive flow from the LAD, dysfunctional (stenosis >70%, sub-occlusion or occlusion).
Because LIMA graft flow is dependent on the LAD and the left subclavian artery flow, we also analyzed the aspect of the LAD after the anastomosis with LIMA graft and the left subclavian artery. LAD flow after the anastomosis with LIMA graft was noted as follows: native-dominant flow, in which the distal LAD was well visualized from the native coronary injection; balanced flow, in which the distal LAD was visualized faintly; graft-dominant flow, in which the distal LAD was not visualized from the native coronary injection.
Statistical analysis
Data are presented as mean ± SD for continuous variables and as number and percentage for dichotomial variables. The analysis was performed using SPSS 20.0 (Statistical Package for Social Sciences version 20, SPSS, Inc, Chicago, Illinois, USA). The Spearman correlation coefficient was used to determine univariate relations. Sensitivity, specificity and diagnostic accuracy were calculated and compared using the chi-square test results. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) were calculated, with 95% confidence intervals. A probability of type I error of 5% was established for every analysis, the value of p <0.05 being considered statistically significant.
To establish cut-off values for the TTE parameters to assess LIMA graft patency, we used the mathematical model “fixed threshold but different values” to classify patent/ dysfunctional grafts. From this model we obtained classification thresholds, with minimum and maximum values. The medical interpretation was the following:
- patent LIMA (the values for both parameters were greater than their maximum thresholds)
- dysfunctional LIMA (the values for both parameters were smaller than their minimum thresholds)
- values could not be medically interpreted (the values of the parameters were between their minimum and maximum threshold values)
Cut-off values were determined by selecting points of potential clinical value, based on the literature data9,14,15, namely a DSVR ratio ≥0.5 or <0.5 and a DF ≥50% or <50%, for patent, and for dysfunctional grafts, respectively. Those 2 parameters had not been previously analyzed together, only separately15.
RESULTS
General characteristics of the study population (Tabel 1)
The majority of patients were men (85%), with a mean age of 64 ± 10 (mean ± standard deviation: SD). The number of grafts for each patient was 2 for 44% of them, 3 for 30% of them, the others having 1 or 4 grafts. The mean duration of grafts was 7 ± 4.53 years, most of them being older than 5 years (49%). 7% of patients were still smoking, all were hypertensive, 44% were diabetics and 33% still had uncontrolled hypercholesterolemia.
Table 1. General characteristics of the study population
| Crt. No. | Variable | Percent of patients/Number ± SD |
| 1. | Male sex | 85% |
| 2. | Mean Age | 64 ± 10 years |
| 3. | Number of grafts/patient 2 grafts 3 grafts 1 or 4 grafts |
44% 30% 36% |
| 4. | Mean duration of grafts | 7 ± 4.5 years |
| 5. | Still smoker patients | 7% |
| 6. | Hypertensive patients | 100% |
| 7. | Diabetic patients | 44% |
| 8. | Uncontrolled hypercolesterolemia | 33% |
Transthoracic Doppler echocardiography and angiography of the LIMA graft
We detected LIMA graft in 29 out of 30 patients (96.66%), from the left parasternal window.
None of the ACS had the LIMA graft as the culprit vessel. In our study, all LIMA grafts were bridged to the LAD and the patency rate for the LIMA at 7 ± 4.53 years after CABG was 93.33%, only 2 (6.66%) of them with body sub-occlusion.
Two patients (6.66%) had a LIMA graft body sub-occlusion, one had a LIMA graft with competitive flow from the LAD and the other with a 30% stenosis at distal anastomosis with native LAD.
Only in one patient did the left subclavian artery have a 50% proximal stenosis, all other 29 patients had left subclavian arteries without stenosis. Even if the left subclavian artery had a 50% stenosis, the flow in the LIMA graft had not been influenced yet and we decided to follow the left subclavian artery every 3 months by ultrasound.
Patient number 6 (Figure 3) had a partial systolic reversal flow (SRF) showing in a TTE from the 3rd intercostal space window, with a DSVR of 1.18 and a DF of 67.13%. An angiography showed that the LIMA was still patent, with competitive flow from the native LAD, which was without significant stenosis.
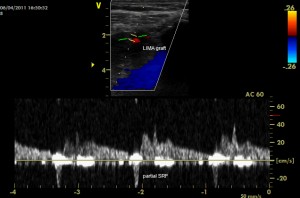
Figure 3. Partial systolic reversal flow showed by TTE from the 3rd intercostal space window in patient number 6.
Patient number 23 (Figure 4) and patient number 29 (Figure 5) had a total SRF showing in a TTE from the 4th intercostal space, with a DSVR <0.5 and a DF <50%. The angiographic examination showed that the LIMA had a body subocclusion. The native LAD did not have any significant stenosis in neither of the two patients, and the flow in the LAD was native-dominant.
Patient number 28 had a 30% distal stenosis of the LIMA and a native LAD with balanced flow. TTE results showed that he had a DSVR of 0.48 and a DF of 48%.
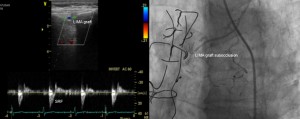
Figure 4. Total systolic reversal flow showed by TTE (left) and LIMA graft body subocclusion showed by angiography (right) in patient number 23.
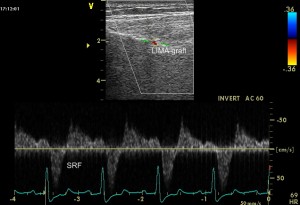
Figure 5. Total systolic reversal flow showed by TTE from the left intercostal space in patient number 29.
In 3 (10%) patients we have obtained SRF (one partial and two totals), a particular flow in the LIMA graft that usually occurs due to competitive flow with the native coronary artery. All three native LAD were without significant stenosis. The LIMA graft was evaluated 5, 7 and 10 years respectively after CABG. In patient number 6, 5 years after the CABG, a TTE showed that the LIMA was still patent, with competitive flow with the LAD, with a DSVR of 1.18 and a DF of 67.13%. In patient number 23 and patient number 29 respectively, 7 years and 11 years respectively after the CABG, a TTE showed that the LIMA was already dysfunctional, with DSVR <0.5 and DF <50% in both cases.
Correlations of echocardiographic and angiographic results for the LIMA grafts
In our study, the DF (p=0.016) and the presence of systolic reversal flow (p=0.0001) are statistically significant, correlated with the angiographic aspect of the LIMA graft, while the DSVR ratio is almost statistically significant correlated with the angiographic aspect of the LIMA graft (p=0.059).
Using the ROC curve analysis (Figure 6) we have obtained the following threshold values for DSVR and DF, as noted in Table 2: DSVR ratio minimum=0.32 and maximum=0.50; DF minimum=49% and maximum=51%.
Table 2. Medical interpretation for DSVR ratio and DF to assess LIMA graft patency
| TDE of the LIMA graft from intercostals space window | Diastolic Fraction | |||
| <49% | 49-51% | ≥51% | ||
| DSVR | <0.32 | dysfunctional | dysfunctional | not interpretable |
| 0.32-0.50 | dysfunctional | not interpretable | patent | |
| ≥0.50 | not interpretable | patent | patent | |
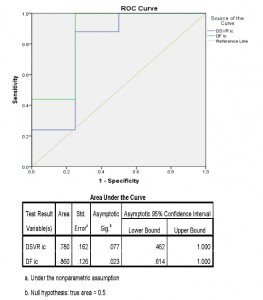
Figure 6. ROC curve to determine the best cutoff points for the DSVR and the DF from the left intercostal window in evaluating the patency of LIMA grafts.
A DSVR <0.32 and a DF <49% identify a dysfunctional LIMA graft with a sensitivity of 50% and 75% respectively and a 100% specificity. A DSVR ≥0.5 and a DF ≥51% identify a patent LIMA graft with 75% sensitivity and 88% specificity. When the obtained values for DSVR (between 0.32 and 0.50) and DF (between 49% and 51%) are between their threshold values, we can confirm neither the patency, nor the dysfunctionality of the graft.
Table 3 contains all the values obtained for the TTE parameters and the coronarography results for all patients.
Table 3. Transthoracic Doppler parameters and coronarography of the LIMA graft and LAD
| Left Parasternal Window | |||||
| No. patient | DSVR | DF(%) | SRF | Coronarography_LIMA | Coronarography_LAD |
| 1 | 0.56 | 57.25 | 0 | patent | graft-dominant |
| 2 | 0.51 | 51.54 | 0 | patent | graft-dominant |
| 3 | 0.97 | 72.5 | 0 | patent | graft-dominant |
| 4 | 0.71 | 73.19 | 0 | patent | graft-dominant |
| 5 | 0.74 | 63.38 | 0 | patent | graft-dominant |
| 6 | 1.18 | 67.13 | 1 | patent, competitive flow from IVA | native dominant, IVA without stenosis |
| 7 | 0.74 | 58.19 | 0 | patent | graft-dominant |
| 8 | 0.52 | 51 | 0 | patent | graft-dominant |
| 9 | 0.93 | 61.03 | 0 | patent | graft-dominant |
| 10 | 1.06 | 71.19 | 0 | patent | graft-dominant |
| 11 | 0.6 | 64.54 | 0 | patent | graft-dominant |
| 12 | 0.65 | 58.08 | 0 | patent | graft-dominant |
| 13 | 0.42 | 49.08 | 0 | patent | graft-dominant |
| 14 | 0.32 | 49.77 | 0 | patent | graft-dominant |
| 15 | NV | NV | NV | patent | graft-dominant |
| 16 | 0.5 | 56.25 | 0 | patent | graft-dominant |
| 17 | 0.74 | 66.67 | 0 | patent | graft-dominant |
| 18 | 1.26 | 73.08 | 0 | patent | graft-dominant |
| 19 | 1.91 | 81.48 | 0 | patent | graft-dominant |
| 20 | 0.46 | 49.4 | 0 | patent | graft-dominant |
| 21 | 0.81 | 67.68 | 0 | patent | graft-dominant |
| 22 | 1.88 | 77.58 | 0 | patent | graft-dominant |
| 23 | -0.59 | 22.29 | 1 | dysfunctional, midgraft subocclusion | native dominant, IVA without stenosis |
| 24 | 1.21 | 67.52 | 0 | patent | graft-dominant |
| 25 | 1.91 | 79.17 | 0 | patent | graft-dominant |
| 26 | 0.98 | 71.39 | 0 | patent | graft-dominant |
| 27 | 1.28 | 72.16 | 0 | patent | graft-dominant |
| 28 | 0.48 | 48 | 0 | 30% distal stenosis | balanced- flow, IVA with serial stenosis |
| 29 | -0.46 | 25 | 1 | dysfunctional, midgraft subocclusion | native dominant, IVA without stenosis |
| 30 | 0.56 | 54 | 0 | patent | graft-dominant |
| DSVR ratio= diastolic/ systolic peak velocity ratio, DF=diastolic fraction, SRF=systolic reversal flow, NV= not visualised | |||||
DISCUSSION
The use of the LIMA graft to revascularize the LAD has become widely adopted in the last decades, so there is a great number of patients with aging grafts who need noninvasive methods to assess graft patency. In recent years, noninvasive detection of coronary artery bypass grafts has been carried out by some studies using the transthoracic Doppler technique5,6,10,11,17.
The results of the present study demonstrate that TTE is able to detect LIMA grafts, as we have obtained a description of the graft in 29 out of 30 patients (96.66%), and the echocardiographic findings are reliable after comparing them with those showed by coronary angiography. The two parameters used to assess graft patency (DSVR and DF) were also used in other studies, each having obtained different values to characterize patent or dysfunctional grafts.
In the study by Hwan Jun et al.17 a DSVR ratio <0.40 predicted significant LIMA graft stenosis with a sensitivity and specificity of 100% and 75% respectively. El-Masry et al.18 concluded that a DSVR ratio <1 predicted 70% LIMA graft stenosis with sensitivity and specificity of 100% and 90%, respectively. In the study by Takagi et al.19, a DSVR ratio <0.6 predicted severe (>75% diameter) LIMA graft stenosis with a sensitivity and specificity of 100% and 80%, respectively. In our study, a DSVR ratio <0.32 identified dysfunctional grafts, with 50% sensitivity and 100% specificity. Their study population consisted of 56 grafts, 11 (19%) with severe stenosis (>75%), while in our study only two grafts (6.66%) had severe stenosis (sub-occlusion).
In the study of Madaric et al.20, a DSVR >0.5 yielded optimal accuracy in detecting the absence of LIMA bypass dysfunction with a negative predictive value of 95%. In our study too, a DSVR ≥0.5 identified patent LIMA grafts, with a negative predictive value of 95.65%. In the study by Hata et al.21, a DSVR ratio >1 was associated with good angiographic findings, without mentioning anything about the degree of any insignificant stenosis of the LIMA graft.
Calafiore et al.22 showed that if the DSVR is ≥1, the LIMA graft is “non-restrictive” and if the DSVR is <0.6, the LIMA graft is probably occluded. They did not mention anything about the interpretation of the DSVR values between 0.6 and 1. The results of our study are consistent with the results obtained by Calafiore et al.22, which means that when the DSVR is between 0.32 and 0.5 the results cannot be accurately interpreted.
In our study, the threshold value for the DSVR which allows the identification of dysfunctional LIMA grafts is smaller than the ones described in the abovementioned studies, less than 0.32. This is probably due to the fact that we only had two LIMA grafts with severe stenosis (subocclusion), without having graft stenosis between 75% and 90%.
Thus, when reading the literature, we will find studies that obtained a DSVR of <118,23,24, other studies obtained a DSVR of <0.55,7,19,23 and one study obtained a DSVR of <0.619., all of which allow the identification of significant LIMA graft stenosis. Which one should we trust and which is the best threshold value for the DSVR in order to identify patent or dysfunctional LIMA grafts? We think those DSVR values are so different because the incidence of LIMA graft dysfunction is quite rare25-28 and each of these studies, as ours too, had a small number of dysfunctional grafts, so statistically, their specificity was great, with poor sensitivity.
The DF expresses how much the diastolic flow represents in relation to the total flow (systolic plus diastolic) and it can be calculated using VTI (cm) or the blood flow (ml/min)10. Almost all published studies in literature used a DF<50% to identify severe stenosis of the LIMA graft at rest, as it is considered the “golden standard criteria” for graft patency5. The DF is thought to be the most powerful predictor because the peak velocity ratio might be affected by native vessel stenosis5,9 and by the aspect of the native LAD after the anastomosis with LIMA graft.
Song et al.8 stated that the ‘occluded’ grafts showed a transthoracic DF of less than 60% in all grafts. n the meta-analysis by Jones et al.15, a DF of less than 50%, with an 89% sensitivity and a 94% specificity was the most accurate criterion to identify LIMA graft stenosis, although the DSRV ratio also performed well. In the study of Pizzuto29, a DF<50% had a 69% diagnostic accuracy predicting LIMA graft stenosis ≥70%. In our study, a DF <49% identified dysfunctional LIMA grafts with 100% specificity and PPV, and a 96.15% NPV. We obtained a DF ≥51% (sensitivity 75%, specificity 88%) which allowed the identification of patent LIMA grafts. Our study is in accordance with the abovementioned studies, as the DF ≥51% identified patent LIMA grafts in our study as well. In what dysfunctional LIMA grafts are concerned, our threshold value for the DF is smaller (<49%) than in other studies, probably due to the fact that, as we have mentioned before, we only had 2 LIMA grafts with body subocclusion in our study population.
When obtaining borderline values, between the minimum and maximum threshold values for any of the parameters (Table 1), we can neither say that the LIMA graft is patent, nor dysfunctional (in our study group, one graft had 30% stenosis at the anastomosis with LAD and the other one had competitive flow with LAD, but was still patent). We would need an alternative method to say with certainty whether the graft is patent or dysfunctional. There are also researchers who believe that it is not clear whether a normal flow pattern during resting conditions necessarily excludes the presence of a critical stenosis6,7,10,11. In the study of Cicala et al.32, the DSVR ratio in multLADriate analysis was not an independent predictor of LIMA graft function. The functional impairment of a vessel is probably better assessed by measuring its flow reserve. The measurement of blood flow after the use of a coronary vasodilator would allow noninvasive assessment of flow reserve in the LIMA and may be useful in the evaluation of graft stenosis of moderate severity. Chirillo et al.33 concluded that in their study none of the baseline Doppler indices could discriminate between patent and stenotic grafts. We believe that performing graft flow reserve when obtaining resting inconclusive TTE parameters would help discriminate patent from dysfunctional LIMA grafts.
The discovery of a SRF should raise some questions about the graft patency and the stenosis of the native coronary artery. In our study, two patients had total systolic reversal of flow, a rare discovery thaw7,15,33,34,35 with LIMA body graft subocclusion and one patient had partial SRF with a still patent LIMA; all three abovementioned patients had the native LAD without significant stenosis. It seems that chronic competitive flow from the native artery is the main cause of the LIMA graft dysfunction7,16,36,37,38,39 as shown by TTE and this is validated by the discovery of the SRF, which can be partial in the early stages, before the LIMA graft becomes dysfunctional, and total when the LIMA graft is already dysfunctional34,39.
Studying resting coronary graft flow by means of transthoracic Doppler echocardiography is noninvasive, can be performed at any time, even before coronary angiography in conditions of acute coronary syndromes and is very challenging for the examiner. It requires accurate interpretation, always taking into consideration the multifactorial context of the patient.
Study limitations
The first important limitation of the study is the size of the population, comprised of only 30 patients, and the second is the small number of dysfunctional LIMA grafts, only two. However, our findings are compatible with previous studies in larger populations31,33 with low incidence of LIMA graft dysfunction, even after more than 10 years after the CABG40.
The third important limitation of our study is the fact that we did not perform LIMA graft flow reserve, we only propose it as an alternative noninvasive method for graft patency, when resting DSVR and DF are not diagnostic.
This study did not obtain data on patients’ comorbidities, the used medications, previous coronary anatomy, nor on the left ventricular systolic function, all of these being factors that could affect LIMA flow.
CONCLUSIONS
Rest TTE parameters of LIMA grafts, a DSVR ≥0.5 and a DF ≥51% can correctly identify patent grafts, while a DSVR <0.32 and a DF <49% identify dysfunctional grafts. The discovery of a SRF should raise some questions about flow competition between LIMA graft and native LAD. Therefore, this totally noninvasive method, the TTE of the LIMA graft, may provide additional information for cardiac catheterization at the bedside or in outpatient clinics, and would also be useful during follow-up of patients with previous CABG.
List of abbreviations
left internal mammary artery (LIMA)
anterior interventricular artery (LAD)
coronary artery bypass graft (CABG)
transthoracic Doppler echocardiography (TTE)
acute coronary syndromes (ACS)
peak systolic velocity (PSV )
peak diastolic velocity (PDV)
systolic and diastolic velocity time integral (SVTI and DVTI)
peak diastolic to systolic velocity ratio (DSVR)
diastolic fraction (DF)
positive predictive value (PPV)
negative predicteve value (NPV)
systolic reversal flow (SRF)
Competing interests: The authors declare that they have no competing interests.
Authors’ contributions: All authors have contributed to the manuscript and approved the final version.
References
1. Loop F, Lytle B, Cosgrove D, Stewart R, Goormastic M, Williams G, Golding L, Gill C, Taylor P, Sheldon W, Proudfit W. Influence of the internal mammary artery graft on 10-year survLADl and other cardiac events. N Engl J Med 1986; 314:1–6.
2. Hartman J.M., Kelder J.C., Ackerstaff R.G.A., Vermeulen F.E.E., Bogers A.J.J.C. Differences in LIMA Doppler characteristics for different LAD perfusion areas. Eur J Cardiothorac Surg 2001; 20: 1135-1141.
3. Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation 1995; 92: 2333–42.
4. Crowley JJ, Shapiro LM. Noninvasive assessment of left internal mammary artery graft patency using transthoracic echocardiography: Circulation 1995; 92 (Suppl II): II25–30.
5. Pezzano A, Fusco R, Child M. Assessment of left internal mammary artery grafts using dipyridamole Doppler echocardiography. Am J Cardiol 1997; 80: 1603–6.
6. Akasaka T, Yoshida K, Hozumi T, Takagi T, Kaji S, Kawamoto T, Morioka S, Nasu M and J Yoshikawa. Flow dynamics of angiographically no-flow patent internal mammary artery grafts. J Am Coll Cardiol 1998; 31:1049-56.
7. Pizzuto F, Voci P, Mariano E, Puddu PE, Chiavari PA, Romeo F. Noninvasive coronary flow reserve assessed by transthoracic coronary Doppler ultrasound in patients with left anterior descending coronary artery stents. Am J Cardiol 2003; 91:522–6.
8. Song MH, Ito M, Toki S, Tanaka S, Kato W, Iwase J, Tajima K. Echocardiographic Evaluation of Internal Mammary Artery Graft Patency. Asian Cardiovasc Thorac Ann 2004; 12:130–132.
9. Leitão MC, Filho JGL, Freire TM, Montenegro ML, Jamacuru FVH, de Carvalho ER, Ximenes Couto Bem A, Lobo Filho HG, de Moraes Filho MO. Doppler echocardiographic criteria in patency assessment of composite grafts from left internal thoracic artery. Rev Bras Cir Cardiovasc 2013; 28 (2):167-75.
10. Fukata Y, Horike K, Fujimoto E, Shimoe Y, Kanbara T. Evaluation of the internal thoracic arterial graft patency by the transthoracic Doppler method under continuous intravenous infusion of adenosine triphosphate disodium. Ann Thorac Cardiovasc Surg 1999; 5: 310–20.
11. Voci P, Plaustro G, Testa G, Marino B, Campa PP. Visualizzazione delle arterie mammarie interne native e del graft aortocoronarico con ecocardiografia color-Doppler ad alta risoluzione. Cardiologia 1998; 43:403–6.
12. Pagni S, Storey J, Ballen J, Montgomery W, Chiang BY, Etoch S, Spence PA. ITA versus SVG: a comparison of instantaneous pressure and flow dynamics during competitive flow. Eur J Cardiothorac Surg 1997; 11:1086-92.
13. Pepine et al. ACC/AHA Guidelines for Cardiac Catheterization and Cardiac Catheterization Laboratories. JACC 1991; 5 (18): 1149-82.
14. Bharat D, Bhan A, Choudhary SK, Sharma S, Sharma R, Airan B and Venugopal P. Assessment of Internal Mammary Artery Graft Patency: Angiography or Doppler? Asian Cardiovasc Thorac Ann. 2000; 8: 325-329.
15. Jones C, Athanasiou T, Paris P, Malinovski V, Purkayastha S, Haq A, Kokotsakis J, Darzi A. Does Doppler echography have a diagnostic role in patency assessment of internal thoracic artery grafts? Eur J Cardiothorac Surg. 2005; 28: 692-700.
16. Kawasuji M, Sakakibara N, Takemura H, Tedoriya T, Ushijima T, Watanabe Y. Is internal thoracic artery grafting suitable for a moderate stenotic coronary artery ? J Thorac Cardiovasc Surg 1996; 112:253- 257.
17. Hwan Jun J, Young Hwan K, Hyung Beom K, Ki Bong K, Jin Wook C, Jae Hyung P. Evaluation of the Internal Mammary Artery Graft: Usefulness of Transcutaneous Doppler Sonography. Journal of Korean Society of Ultrasound in Medicine 2000; 19 (1):51-56.
18. El-Masry MM, Salama MM, Darwish AZ, Aziz O. Assessment of Left Internal Mammary Artery Graft Patency by Transthoracic Doppler Echocardiography. Clin. Cardiol. 2002; 25:511–516.
19. Takagi T, Yoshikawa J, Yoshida K, Akasaka T, Maeda K. Noninvasive measurement of left internal mammary artery bypass graft flow by duplex Doppler echocardiography from the supraclavicular fossa. J Am Soc Echocardiogr 1993; 6:374-81.
20. Madaric J, Mistrik A, Riecansky I, Vulev L, Pacak J, Verhamme K, De Bruyne B, Fridrich V and Bartunek J. Left internal mammary artery bypass dysfunction after revascularization of moderately narrowed coronary lesions. Colour-duplex ultrasound versus angiography study. European Journal of Echocardiography 2008; 9:273-277.
21. Hata M, Raman JS, Shiono M, Sezai A, Negishi N, Sezai Y, Seevanayagam S, Kanagasaby R, Store M, Croce ED and Buxton BF. What can Doppler Wave Forms of the Left Internal Thoracic Artery Teach Us? The Efficacy of Apical Transthoracic Approach of Doppler Echocardiography. Ann Thorac Cardiovasc Surg 2002; 8:92-6
22. Calafiore AM, Gallina S, Iaco A, Teodori G, Iovino T, Di Giammarco G, Mazzei V and Vitolla G. Minimaly invasive mammary artery Doppler flow velocity evaluation in minimally invasive coronary operations. Ann Thorac Surg 1998; 66:1236-41.
23. Kamiya H, Nishimura F, Fukuyama K. Doppler echography from supraclavicular fossa—assessment of internal mammary artery bypass graft patency. Nippon Rinsho 1994; 52 (Suppl. Pt 1): 327-32.
24. De Simone L, Caso P, Severino S, Cicala S, Galderisi M, Renzulli A, Bonzani G, Scherillo M, Mininni N, Cotrufo M. Noninvasive assessment of left and right internal mammary artery graft patency using transthoracic color Doppler echocardiography. Ital Heart J 2003; 4(3):173-8.
25. Naoko M, Kisanuki A, Hamasaki S, Takasaki K, Yuasa T, Kuwahara E, Ueya N, Horizoe Y, Chaen H. Different Flow Patterns Between Left and Right Internal Thoracic Artery Grafts Influence the Evaluation of Severe Graft Stenosis by Transthoracic Doppler Echocardiography. JASE 2011; 24 (7): 768-774.
26. Meimoun P and Tribouilloy C. Non-invasive assessment of coronary flow and coronary flow reserve by transthoracic Doppler echocardiography: a magic tool for the real world. European Journal of Echocardiography 2008; 9:449–457.
27. Katz WE, Zenati M, Mandarino WA, Cohen HA, Gorcsan J III. Assessment of left internal mammary artery graft patency and flow reserve after minimally invasive direct coronary artery bypass. Am J Cardiol 1999; 84:795-801.
28. Shimizu T, Hirayama T, Suesada H, Ikeda K, Ito S, Ishimaru S. Effect of flow competition on internal thoracic artery graft: Postoperative velocimetric and angiographic study. The Journal of Thoracic and Cardiovascular Surgery 2000; 120 (3): 459-465.
29. Pizzuto F, Voci P, Mariano E, Puddu PE, Aprile A, Romeo F. Evaluation of Flow in the Left Anterior Descending Coronary Artery But Not in the Left Internal Mammary Artery Graft Predicts Significant Stenosis of the Arterial Conduit. J Am Coll Cardiol 2005; 45(3):424-32.
30. Gruberg L, Dangas G, Mehran R, Hong MK, Waksman R, Mintz GS, Kent KM, Pichard AD, Satler LF, Lansky AJ, Stone GW, Leon MB. Percutaneous revascularization of the internal mammary artery graft: short- and long-term outcomes. J Am Coll Cardiol 2000; 35:944-948.
31. Berger PB, Alderman EL, Nadel A, Schaff HV. Frequency of early occlusion and stenosis in a left internal mammary artery to left anterior descending artery bypass graft after surgery through a median sternotomy on conventional bypass: benchmark for minimally invasive direct coronary artery bypass. Circulation 1999; 100:2353-8.
32. Cicala S, Renzulli A, Galderisi M, de Simone L, De Feo M, Onorati F, Cerasuolo F, Bonzani G, Caso P, Cotrufo M. Transthoracic Doppler echocardiography of mammary artery grafts to assess graft function. Can J Cardiol 2005; 21(1):45-49.
33. Chirillo F, Bruni A, Balestra G, Cavallini C, Olladri Z, Thomas JD, StritoniP. Assessment of internal mammary artery and saphenous vein graft patency and flow reserve using transthoracic Doppler echocardiography. Heart 2001; 86:424-431.
34. Berger A, MacCarthy PA, Vanermen H, De Bruyne B. Occlusion of Internal Mammary Grafts: a review of the Potential Causative Factors. Acta chir belg 2004; 104:630-634.
35. de Abreu JS, Pinheiro Diógenes TC, de Castro Abreu AL, Façanha Barreto JE, Bonfim de Morais JM, Benevides de Abreu ME, Azevedo Pinto JH, Paes Júnior JN. Internal Thoracic Artery Graft (ITAG): Patency and Functional Status at Rest and During Dobutamine-Stress Echocardiography. Arq Bras Cardiol 2008; 90(1):36-43.
36. Pizzuto F, Voci P, Mariano E, Puddu PE, Spedicato P, Romeo F. Coronary flow reserve of the angiographically normal left anterior descending coronary artery in patients with remote coronary artery disease. Am J Cardiol 2004; 94:577-82.
37. Pârv A, Ober C, Bindea D, Duncea C. Transthoracic Doppler echocardiography of the left internal mammary artery graft: Med Ultrason 2013; 15(1): 45-50.
38. Segal J, Lundergan CF. Determination of the hemodynamic significance of coronary artery stenoses of intermediate severity. Am Heart J 1992; 124: 1073-7.
39. Pârv A, Anchidin O, Ober C, Bindea D, Rãdulescu D, Duncea C, Cãpâlneanu R: Left internal mammary artery graft disfunction diagnosed by transthoracic Doppler echocardiography. A report of two cases. Med Ultrason 2012; 14 (4): 348-351.
40. Tatoulis J, Buxton BF, Fuller JA : Patencies of 2127 arterial to coronary conduits over 15 years. Ann Thorac Surg. 2004;77 (1): 93-101.
 This work is licensed under a
This work is licensed under a